Virus-like-particle based technologies

Our research
Our technology-oriented research is based on the use of recombinant virus-like particles (VLPs), which can be produced by co-expressing the structural proteins of the respective virus. These VLPs can mimic the shells of real viruses very authentically, but are not infectious themselves, which enables research and use outside of laboratories with higher safety levels.
Such authentic VLPs can be used directly as vaccines, one example being the already approved Cervix vaccine against human papillomaviruses. We will research and develop further VLPs as potential vaccine candidates against infectious diseases. At the same time, authentic VLPs serve as a diagnostic tool to evaluate the immune response, e.g., in reaction to a vaccination or the spread of infectious diseases, which is particularly relevant in the context of climate change in terms of the One Health approach.
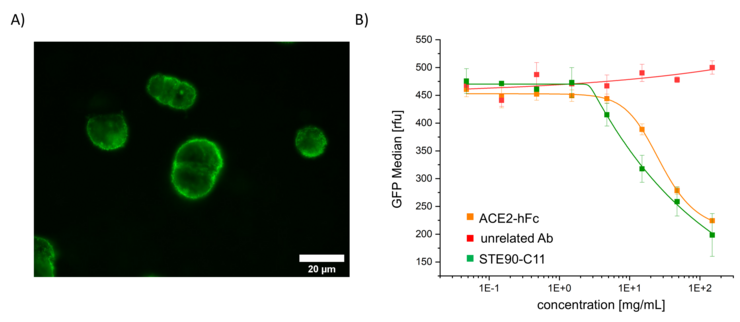
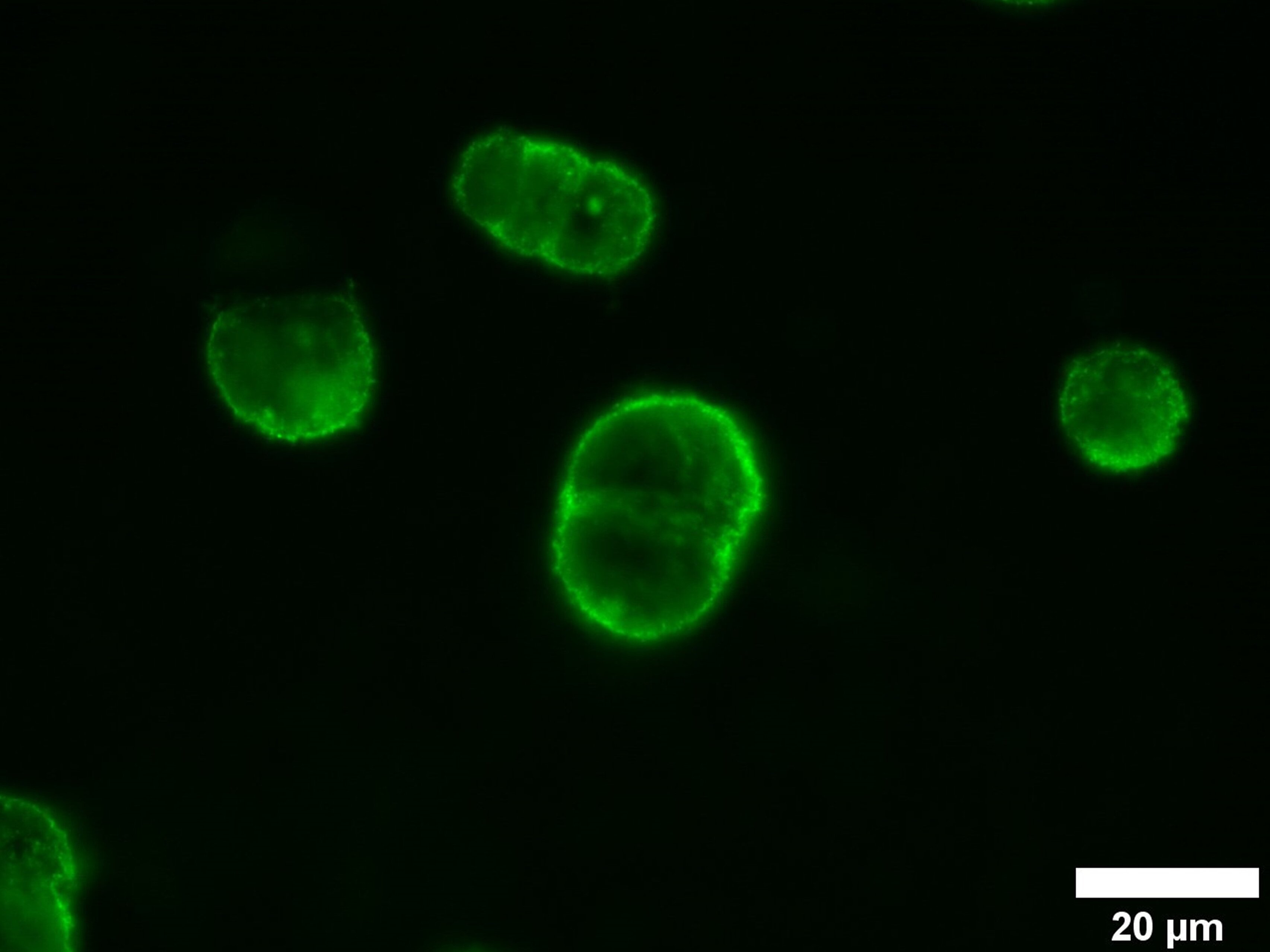
In addition, modified VLPs are important tools for basic research. Modifications inside the VLP, such as the introduction of a fluorescent protein that does not alter the authentic VLP surface, enable investigations of infection mechanisms. These VLPs can also be used for drug development, as the inhibition of binding and fusion of modified VLPs and cells by a drug can be measured and quantified in high-throughput process (Figure 1). Drugs like neutralizing antibodies can thus be analyzed quickly, effectively, and in laboratories with a lower safety level, which is an important step in terms of pandemic resilience.
In addition, we want to generate recombinant VLPs that, thanks to modifications, are able to deliver drugs into cells in a targeted and particularly efficient manner, what still poses a major challenge for various therapeutic approaches. Here, we also aim to develop solutions for latent viral infections.
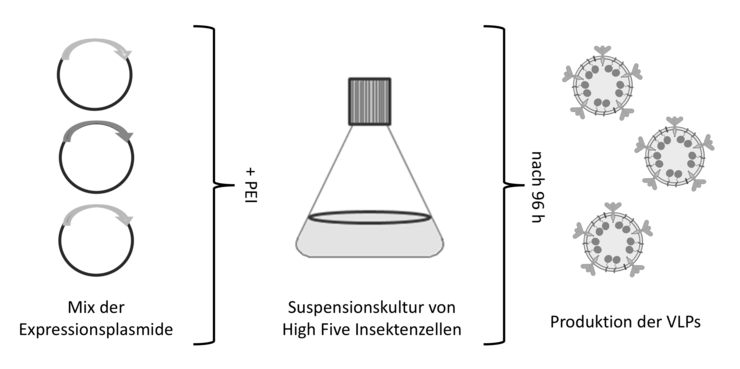
To this end, we mainly use plasmid-based expression in insect cells (Figure 2), a highly efficient system for the production of VLPs (Lampinen et al. 2024). Compared to the baculoviral systems commonly used for insect cells, cell vitality remains high throughout the production process and no baculoviral particles and proteins are produced in parallel to the recombinant VLPs, which improves VLP quality. In addition, the system is very flexible and allows for easy adaptation of individual proteins and production rates by exchanging the respective plasmids.
Our research
Our technology-oriented research is based on the use of recombinant virus-like particles (VLPs), which can be produced by co-expressing the structural proteins of the respective virus. These VLPs can mimic the shells of real viruses very authentically, but are not infectious themselves, which enables research and use outside of laboratories with higher safety levels.
Such authentic VLPs can be used directly as vaccines, one example being the already approved Cervix vaccine against human papillomaviruses. We will research and develop further VLPs as potential vaccine candidates against infectious diseases. At the same time, authentic VLPs serve as a diagnostic tool to evaluate the immune response, e.g., in reaction to a vaccination or the spread of infectious diseases, which is particularly relevant in the context of climate change in terms of the One Health approach.
In addition, modified VLPs are important tools for basic research. Modifications inside the VLP, such as the introduction of a fluorescent protein that does not alter the authentic VLP surface, enable investigations of infection mechanisms. These VLPs can also be used for drug development, as the inhibition of binding and fusion of modified VLPs and cells by a drug can be measured and quantified in high-throughput process (Figure 1). Drugs like neutralizing antibodies can thus be analyzed quickly, effectively, and in laboratories with a lower safety level, which is an important step in terms of pandemic resilience.
In addition, we want to generate recombinant VLPs that, thanks to modifications, are able to deliver drugs into cells in a targeted and particularly efficient manner, what still poses a major challenge for various therapeutic approaches. Here, we also aim to develop solutions for latent viral infections.
To this end, we mainly use plasmid-based expression in insect cells (Figure 2), a highly efficient system for the production of VLPs (Lampinen et al. 2024). Compared to the baculoviral systems commonly used for insect cells, cell vitality remains high throughout the production process and no baculoviral particles and proteins are produced in parallel to the recombinant VLPs, which improves VLP quality. In addition, the system is very flexible and allows for easy adaptation of individual proteins and production rates by exchanging the respective plasmids.
Recombinant Virus-Like-Particles are a versatile and valuable technology to investigate and fight infectious diseases.

Dr. Maren Schubert (née Bleckmann) studied biotechnology at the TU Braunschweig and obtained her PhD at the HZI in 2016 in the group for Recombinant Protein Expression. During her PhD, she developed a novel baculovirus-free expression system for insect cells as well as cell-based screening assays. She then became head of the recombinant protein expression platform at the Rudolf Virchow Centre in Würzburg, where she was responsible for the production of various proteins for different projects. In 2018, she returned to the TU Braunschweig to work on antigen and antibody expression systems and the development of cellular assays in the Department of Biotechnology. The SARS-CoV-2 antigens produced in her insect cell system were applied e.g. for the development of anti-COVID-19 antibodies (COR101, NCT04674566) and in various diagnostic studies. In addition, she developed virus-like particle (VLP)-based systems for the generation, evaluation and development of monoclonal antibodies. In September 2025, she is now back at the HZI as HUMAN junior research group leader and focuses on VLP-based technologies to successfully fight infectious diseases.