Author count: 1
Rox K.
(2026)
Exploiting Pharmacokinetic/Pharmacodynamic Methods for Optimizing and Accelerating Drug Development of Innovative Anti-Infectives
ChemMedChem.,
21
(2)
Author count: 2
Metzen A., Rox K.
(2025)
A Streamlined High Performance Liquid Chromatography with Tandem Mass Spectrometry Based Workflow for Rapid Screening of Cellular Accumulation of Small Molecules
ChemMedChem
Author count: 14
Buffa V., Kowalewski J., Qi G., Deutscher R., Cica M., Richardoz M., Tomaszczyk M., Krämer A., Knapp S., Dunyach-Remy C., Rox K., Guichou J.F., ... , Lionne C., Hausch F.
(2025)
Targeting bacterial kinases as a strategy to counteract antibiotic resistance
Commun.Chem.,
8
(1)
Author count: 9
Friederich J., Rox K., Fuchs H.L.S., Hasan M.M., Raunft P., Thurston D.E., Fox K.R., ... , Rahman K.M., Brönstrup M.
(2025)
Synthesis and Evaluation of DNA Cross-linkers by Click Chemistry-Mediated Heterodimerization of Nor-Tomaymycins
Chemistry - A European Journal
Author count: 55
Cunningham C.E., Vizeacoumar F.S., Zhang Y., Kyrylenko L., Both S., Maranda V., Dong H., Price J.D.W., Gao P., Wagner K., Wu Y., Lazell-Wright M., Ganapathysamy A., Hari R., Bhanumathy K.K., Denomy C., Saxena A., Vizeacoumar J.P., Morales A.M., Khan F., Mosley S., Chen A., Katrii T., Zoller B.G.E., Rajamanickam K., Walke P., Gong L., Patel H., Elhasasna H., Dahiya R., Abuhussein O., Dmitriev A., Freywald T., Munhoz E.P., Ruppin E., Lee J.S., Rox K., Koebel M., Hopkins L., Lee C.H., Yadav S., Gasparoni G., Walter J., Krishnan A., Datla R., Toosi B., Baker K., Meens J., Cescon D.W., Ailles L., Leary S.C., Empting M., Kiemer A.K., ... , Freywald A., Vizeacoumar F.J.
(2025)
Identification of targetable vulnerabilities of PLK1-overexpressing cancers by synthetic dosage lethality
Cell Genomics,
5
(6)
Author count: 24
Boudrioua A., Joiner J.D., Grin I., Kronenberger T., Korotkov V.S., Steinchen W., Kohler A., Schminke S., Schulte J.C., Pietsch M., Naini A., Kalverkamp S., Hotop S.K., Coyle T., Piselli C., Coles M., Rox K., Marschal M., Bange G., Flieger A., Poso A., Brönstrup M., ... , Hartmann M.D., Wagner S.
(2025)
Discovery of synthetic small molecules targeting the central regulator of Salmonella pathogenicity
Science advances,
11
(15)
Author count: 40
Shekhar A., Di Lucrezia R., Jerye K., Korotkov V.S., Harmrolfs K., Rox K., Weich H.A., Ghai I., Delhommel F., Becher I., Degenhart C., Fansa E., Unger A., Habenberger P., Klebl B., Lukat P., Schmelz S., Henke S., Borgert S., Lang J.C., Sasse F., Diestel R., Richter C., Schneider-Daum N., Hinkelmann B., Niemz J., Lehr C.M., Jänsch L., Huehn J., Alm R., Savitski M., Welte T., Hesterkamp T., Sattler M., Winterhalter M., Blankenfeldt W., Medina E., Bilitewski U., ... , Dinkel K., Brönstrup M.
(2025)
Highly potent quinoxalinediones inhibit a[alpha]-hemolysin and ameliorate Staphylococcus aureus lung infections
Cell Host and Microbe,
33
(4)
Author count: 13
Sheldon J.A., Winkler M., Yuan Q., Frericks N., Phillip Brown R.J., Miskey C., Gödecke N., Behme S., Rox K., Mysegades G., Vondran F., ... , Wirth D., Pietschmann T.
(2025)
Adapted hepatitis C virus clone infects innate immunity-deficient mouse hepatocytes with minimal human HCV entry factors
JHEP Reports,
7
(5)
Author count: 23
Kiefer A.F., Schütz C., Englisch C.N., Kolling D., Speicher S., Kany A.M., Shafiei R., Wadood N.A., Aljohmani A., Wirschem N., Jumde R.P., Klein A., Sikandar A., Park Y.M., Krasteva-Christ G., Yildiz D., Abdelsamie A.S., Rox K., K+Âhnke J., M++ller R., Bischoff M., ... , Haupenthal J., Hirsch A.K.H.
(2025)
Dipeptidic Phosphonates: Potent Inhibitors of Pseudomonas aeruginosa Elastase B Showing Efficacy in a Murine Keratitis Model
Advanced Science
Author count: 10
Charoenpattarapreeda J., Tegge W., Xu C., Harmrolfs K., Hinkelmann B., Wullenkord H., Hotop S.K., Beutling U., ... , Rox K., Brönstrup M.
(2024)
A Targeted Click-to-Release Activation of the Last-Resort Antibiotic Colistin Reduces its Renal Cell Toxicity
Angew.Chem.Int.Ed.,
63
(47)
Author count: 16
Akula R.K., El Kilani H., Metzen A., Röske J., Zhang K., Göhl M., Arisetti N., Marsh G.P., Maple H.J., Cooper M.S., Karadogan B., Jochmans D., Neyts J., Rox K., ... , Hilgenfeld R., Brönstrup M.
(2025)
Structure-Based Optimization of Pyridone a(alpha)-Ketoamides as Inhibitors of the SARS-CoV-2 Main Protease
J.Med.Chem.
Author count: 21
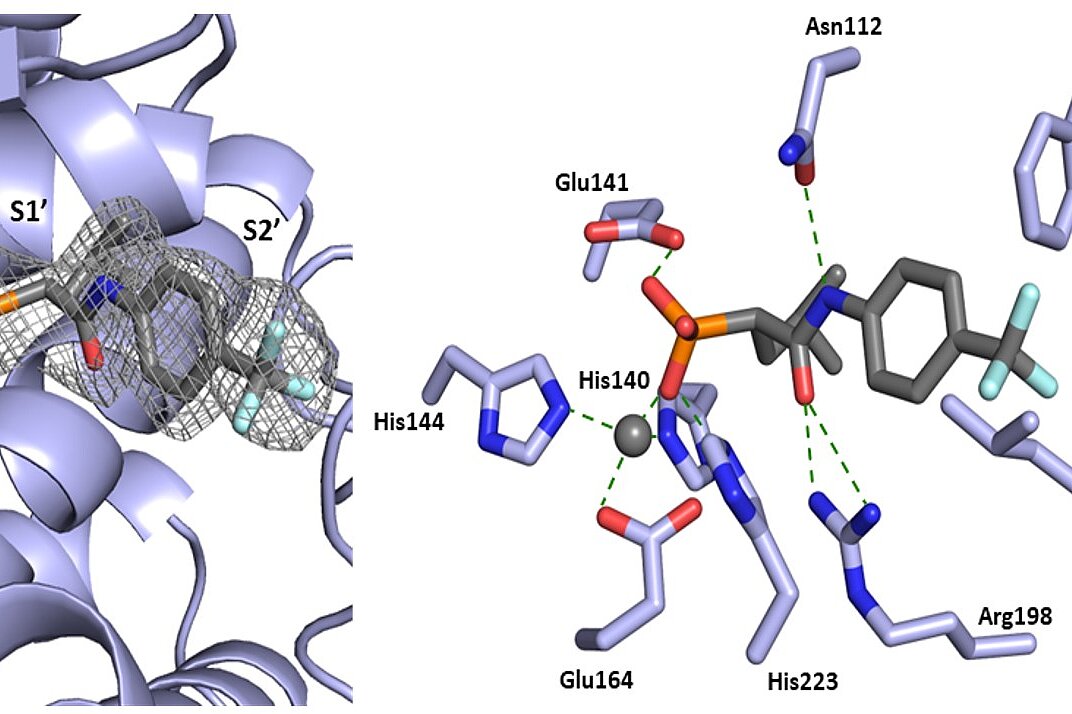
Flury P., Krüger N., Sylvester K., Breidenbach J., Al Hamwi G., Qiao J., Chen Y., Rocha C., Serafim M.S.M., Barbosa da Silva E., Pöhlmann S., Poso A., Kronenberger T., Rox K., O'Donoghue A.J., Yang S., Sträter N., Gütschow M., Laufer S.A., ... , Müller C.E., Pillaiyar T.
(2025)
Design, Synthesis, and Unprecedented Interactions of Covalent Dipeptide-Based Inhibitors of SARS-CoV-2 Main Protease and Its Variants Displaying Potent Antiviral Activity
J.Med.Chem.
Author count: 2
Mishra S., Rox K.
(2024)
Effective, but Safe? Physiologically Based Pharmacokinetic (PBPK)-Modeling-Based Dosing Study of Molnupiravir for Risk Assessment in Pediatric Subpopulations
ACS Pharm.Transl.Sci.,
7
(12)
Author count: 12
Stappert M., Kohnhäuser D., Seedorf T., Coetzee J., Rox K., Fuchs H.L.S., Cirnski K., Leitner C., Herrmann J., Kirschning A., ... , M++ller R., Br+Ânstrup M.
(2024)
Synthetic studies on the tetrasubstituted D-ring of cystobactamids lead to potent terephthalic acid antibiotics
Communications Chemistry,
7
(1)
Author count: 4
Vogel C., Rox K., Wagenlehner F., Titz A.
(2024)
Glycomimetics as Candidates for Treatment and Prevention of Catheter-associated Biofilms Formed by Pseudomonas aeruginosa
Eur.Urol.Focus
Author count: 17
Willocx D., Bizzarri L., Alhayek A., Kannan D., Bravo P., Illarionov B., Rox K., Lohse J., Fischer M., Kany A.M., Hahne H., Rottmann M., Witschel M., Odom John A., Hamed M.M., ... , Diamanti E., Hirsch A.K.H.
(2024)
Targeting Plasmodium falciparum IspD in the Methyl-d-erythritol Phosphate Pathway: Urea-Based Compounds with Nanomolar Potency on Target and Low-Micromolar Whole-Cell Activity
J.Med.Chem.,
67
(19)
Author count: 13
Mielniczuk S., Hoff K., Baselious F., Li Y., Haupenthal J., Kany A.M., Riedner M., Rohde H., Rox K., Hirsch A.K.H., Krimm I., ... , Sippl W., Holl R.
(2024)
Development of Fragment-Based Inhibitors of the Bacterial Deacetylase LpxC with Low Nanomolar Activity
J.Med.Chem.,
67
(19)
Author count: 17
Kohnhäuser D., Seedorf T., Cirnski K., Heimann D., Coetzee J., Sordello S., Richter J., Stappert M., Sabuco J.F., Corbett D., Bacque E., Rox K., Herrmann J., Vassort A., Müller R., ... , Kirschning A., Brönstrup M.
(2024)
Optimization of the Central a[alpha]-Amino Acid in Cystobactamids to the Broad-Spectrum, Resistance-Breaking Antibiotic CN-CC-861
J.Med.Chem.
Author count: 11
Rox K., Kühne A., Herrmann J., Jansen R., Hüttel S., Bernecker S., Hagos Y., Brönstrup M., Stadler M., ... , Hesterkamp T., Müller R.
(2024)
Interaction of the Atypical Tetracyclines Chelocardin and Amidochelocardin with Renal Drug Transporters
ACS Pharm.Transl.Sci.
Author count: 21
Flury P., Breidenbach J., Krüger N., Voget R., Schäkel L., Si Y., Krasniqi V., Calistri S., Olfert M., Sylvester K., Rocha C., Ditzinger R., Rasch A., Pöhlmann S., Kronenberger T., Poso A., Rox K., Laufer S.A., Müller C.E., ... , Gütschow M., Pillaiyar T.
(2024)
Erratum: Cathepsin-Targeting SARS-CoV-2 Inhibitors: Design, Synthesis, and Biological Activity
ACS Pharmacology and Translational Science,
7
(4)
Author count: 2
Rox K., Medina E.
(2024)
Aerosolized delivery of ESKAPE pathogens for murine pneumonia models
Scientific Reports,
14
(1)
Author count: 32
Sake S.M., Zhang X., Rajak M.K., Urbanek-Quaing M., Carpentier A., Gunesch A.P., Grethe C., Matthaei A., R++ckert J., Galloux M., Larcher T., Le Goffic R., Hontonnou F., Chatterjee A.K., Johnson K., Morwood K., Rox K., Elgaher W.A.M., Huang J., Wetzke M., Hansen G., Fischer N., El+®ou+½t J.F., Rameix-Welti M.A., Hirsch A.K.H., Herold E., Empting M., Lauber C., Schulz T.F., Krey T., ... , Haid S., Pietschmann T.
(2024)
Drug repurposing screen identifies lonafarnib as respiratory syncytial virus fusion protein inhibitor
Nature Communications,
15
(1)
Author count: 21
Flury P., Breidenbach J., Krüger N., Voget R., Schäkel L., Si Y., Krasniqi V., Calistri S., Olfert M., Sylvester K., Rocha C., Ditzinger R., Rasch A., Pöhlmann S., Kronenberger T., Poso A., Rox K., Laufer S.A., Müller C.E., ... , Gütschow M., Pillaiyar T.
(2023)
Cathepsin-Targeting SARS-CoV-2 Inhibitors: Design, Synthesis, and Biological Activity
ACS Pharmacology and Translational Science
Author count: 18
Rox K., Jansen R., Lukezic T., Greweling-Pils M., Herrmann J., Miethke M., Hüttel S., Hennessen F., Abou Fayad A., Holzhausen C., Lundberg C.V., Teague J., Sudarman E., Bülter L., Hesterkamp T., Stadler M., ... , Brönstrup M., Müller R.
(2024)
Pharmacokinetic and pharmacodynamic evaluation of the atypical tetracyclines chelocardin and amidochelocardin in murine infection models
Microbiol.Spectr.,
12
(1)
Author count: 21
Konstantinovic J., Kany A.M., Alhayek A., Abdelsamie A.S., Sikandar A., Voos K., Yao Y., Andreas A., Shafiei R., Loretz B., Schönauer E., Bals R., Brandstetter H., Hartmann R.W., Ducho C., Lehr C.M., Beisswenger C., Müller R., Rox K., ... , Haupenthal J., Hirsch A.K.
(2023)
Inhibitors of the Elastase LasB for the Treatment of Pseudomonas aeruginosa Lung Infections
ACS Cent.Sci.,
9
(12)
Author count: 28
Simonis A., Kreer C., Albus A., Rox K., Yuan B., Holzmann D., Wilms J.A., Zuber S., Kottege L., Winter S., Meyer M., Schmitt K., Gruell H., Theobald S.J., Hellmann A.M., Meyer C., Ercanoglu M.S., Cramer N., Munder A., Hallek M., F+ñtkenheuer G., Koch M., Seifert H., Rietschel E., Marlovits T.C., van Koningsbruggen-Rietschel S., ... , Klein F., Rybniker J.
(2023)
Discovery of highly neutralizing human antibodies targeting Pseudomonas aeruginosa
Cell,
186
(23)
Author count: 16
Graspeuntner S., Koethke K., Scholz C., Semmler L., Lupatsii M., Kirchhoff L., Herrmann J., Rox K., Wittstein K., Käding N., Hanker L.C., Stadler M., Bönstrup M., Müller R., ... , Shima K., Rupp J.
(2023)
Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment
Antibiotics.(Basel),
12
(5)
Author count: 2
Khalid K., Rox K.
(2023)
All Roads Lead to Rome: Enhancing the Probability of Target Attainment with Different Pharmacokinetic/Pharmacodynamic Modelling Approaches
Antibiotics.(Basel),
12
(4)
Author count: 5
Peukert C., Rox K., Karge B., Hotop S.K., Brönstrup M.
(2023)
Synthesis and Characterization of DOTAM-Based Sideromycins for Bacterial Imaging and Antimicrobial Therapy
ACS Infect.Dis.,
9
(2)
Author count: 18
Hamed M.M., Abdelsamie A.S., Rox K., Schütz C., Kany A.M., Röhrig T., Schmelz S., Blankenfeldt W., Arce-Rodriguez A., Borrero-de Acuna J.M., Jahn D., Rademacher J., Ringshausen F.C., Cramer N., Tümmler B., Hirsch A.K.H., ... , Hartmann R.W., Empting M.
(2023)
Towards Translation of PqsR Inverse Agonists: From In Vitro Efficacy Optimization to In Vivo Proof-of-Principle
Adv.Sci.
Author count: 15
Rox K., Becker T., Schiefer A., Grosse M., Ehrens A., Jansen R., Aden T., Kehraus S., König G.M., Krome A.K., Hübner M.P., Wagner K.G., Stadler M., ... , Pfarr K., Hoerauf A.
(2022)
Pharmacokinetics and Pharmacodynamics (PK/PD) of Corallopyronin A against Methicillin-Resistant Staphylococcus aureus
Pharmaceutics,
15
(1)
Author count: 12
Zahorska E., Rosato F., Stober K., Kuhaudomlarp S., Meiers J., Hauck D., Reith D., Gillon E., Rox K., Imberty A., ... , Römer W., Titz A.
(2022)
Neutralizing the Impact of the Virulence Factor LecA from Pseudomonas aeruginosa on Human Cells with New Glycomimetic Inhibitors
Angew.Chem.Int.Ed Engl.
Author count: 1
Rox K.
(2022)
Influence of tramadol on bacterial burden in the standard neutropenic thigh infection model
Sci.Rep.,
12
(1)
Author count: 3
Meiers J., Rox K., Titz A.
(2022)
Lectin-Targeted Prodrugs Activated by Pseudomonas aeruginosa for Self-Destructive Antibiotic Release
J.Med.Chem.,
65
(20)
Author count: 7
Mala P., Siebs E., Meiers J., Rox K., Varrot A., ... , Imberty A., Titz A.
(2022)
Discovery of N-ß-l-Fucosyl Amides as High-Affinity Ligands for the Pseudomonas aeruginosa Lectin LecB
J.Med.Chem.
Author count: 18
Cooper M.S., Zhang L., Ibrahim M., Zhang K., Sun X., Röske J., Göhl M., Brönstrup M., Cowell J.K., Sauerhering L., Becker S., Vangeel L., Jochmans D., Neyts J., Rox K., Marsh G.P., ... , Maple H.J., Hilgenfeld R.
(2022)
Diastereomeric Resolution Yields Highly Potent Inhibitor of SARS-CoV-2 Main Protease
J.Med.Chem.,
65
(19)
Author count: 12
Suerbaum S., Coombs N., Patel L., Pscheniza D., Rox K., Falk C., Gruber A.D., Kershaw O., Chhatwal P., Brönstrup M., ... , Bilitewski U., Josenhans C.
(2022)
Correction for Suerbaum et al., "Identification of Antimotilins, Novel Inhibitors of Helicobacter pylori Flagellar Motility That Inhibit Stomach Colonization in a Mouse Model"
MBio.
Author count: 12
Suerbaum S., Coombs N., Patel L., Pscheniza D., Rox K., Falk C., Gruber A.D., Kershaw O., Chhatwal P., Brönstrup M., ... , Bilitewski U., Josenhans C.
(2022)
Identification of Antimotilins, Novel Inhibitors of Helicobacter pylori Flagellar Motility That Inhibit Stomach Colonization in a Mouse Model
MBio.,
13
(2)
Author count: 12
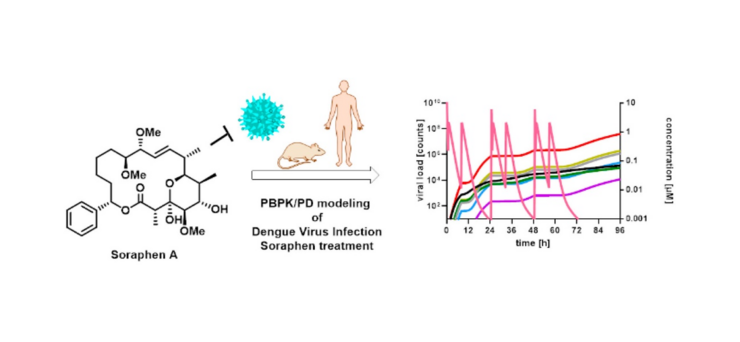
Rox K., Heyner M., Krull J., Harmrolfs K., Rinne V., Hokkanen J., Perez Vilaro G., Díez J., Müller R., Kröger A., ... , Sugiyama Y., Brönstrup M.
(2021)
Physiologically Based Pharmacokinetic/Pharmacodynamic Model for the Treatment of Dengue Infections Applied to the Broad Spectrum Antiviral Soraphen A
ACS Pharm.Transl.Sci.,
4
(5)
Author count: 12
Rath S., Rox K., Kleine Bardenhorst S., Schminke U., Dörr M., Mayerle J., Frost F., Lerch M.M., Karch A., Brönstrup M., ... , Pieper D.H., Vital M.
(2021)
Higher Trimethylamine-N-Oxide Plasma Levels with Increasing Age Are Mediated by Diet and Trimethylamine-Forming Bacteria
mSystems.
Author count: 5
Rox K., Rath S., Pieper D.H., Vital M., Brönstrup M.
(2021)
A simplified LC-MS/MS method for the quantification of the cardiovascular disease biomarker trimethylamine-N-oxide and its precursors
J.Pharmaceut.Analy.
Author count: 26
Schütz C., Ho D.K., Hamed M.M., Abdelsamie A.S., Röhrig T., Herr C., Kany A.M., Rox K., Schmelz S., Siebenbürger L., Wirth M., Börger C., Yahiaoui S., Bals R., Scrima A., Blankenfeldt W., Horstmann J.C., Christmann R., Murgia X., Koch M., Berwanger A., Loretz B., Hirsch A.K.H., Hartmann R.W., ... , Lehr C.M., Empting M.
(2021)
A New PqsR Inverse Agonist Potentiates Tobramycin Efficacy to Eradicate Pseudomonas aeruginosa Biofilms
Adv.Sci.
Author count: 20
Testolin G., Cirnski K., Rox K., Prochnow H., Fetz V., Grandclaudon C., Mollner T., Baiyoumy A., Ritter A., Leitner C., Krull J., van den Heuvel J., Vassort A., Sordello S., Hamed M.M., Elgaher W.A.M., Herrmann J., Hartmann R.W., ... , Müller R., Brönstrup M.
(2020)
Synthetic studies of cystobactamids as antibiotics and bacterial imaging carriers lead to compounds with high: In vivo efficacy
Chem.Sci.,
11
(5)
Author count: 13
Mamareli P., Kruse F., Lu C.W., Guderian M., Floess S., Rox K., Allan D.S.J., Carlyle J.R., Brönstrup M., Müller R., Berod L., ... , Sparwasser T., Lochner M.
(2020)
Targeting cellular fatty acid synthesis limits T helper and innate lymphoid cell function during intestinal inflammation and infection
Mucosal Immunol.,
14
(1)
Author count: 9
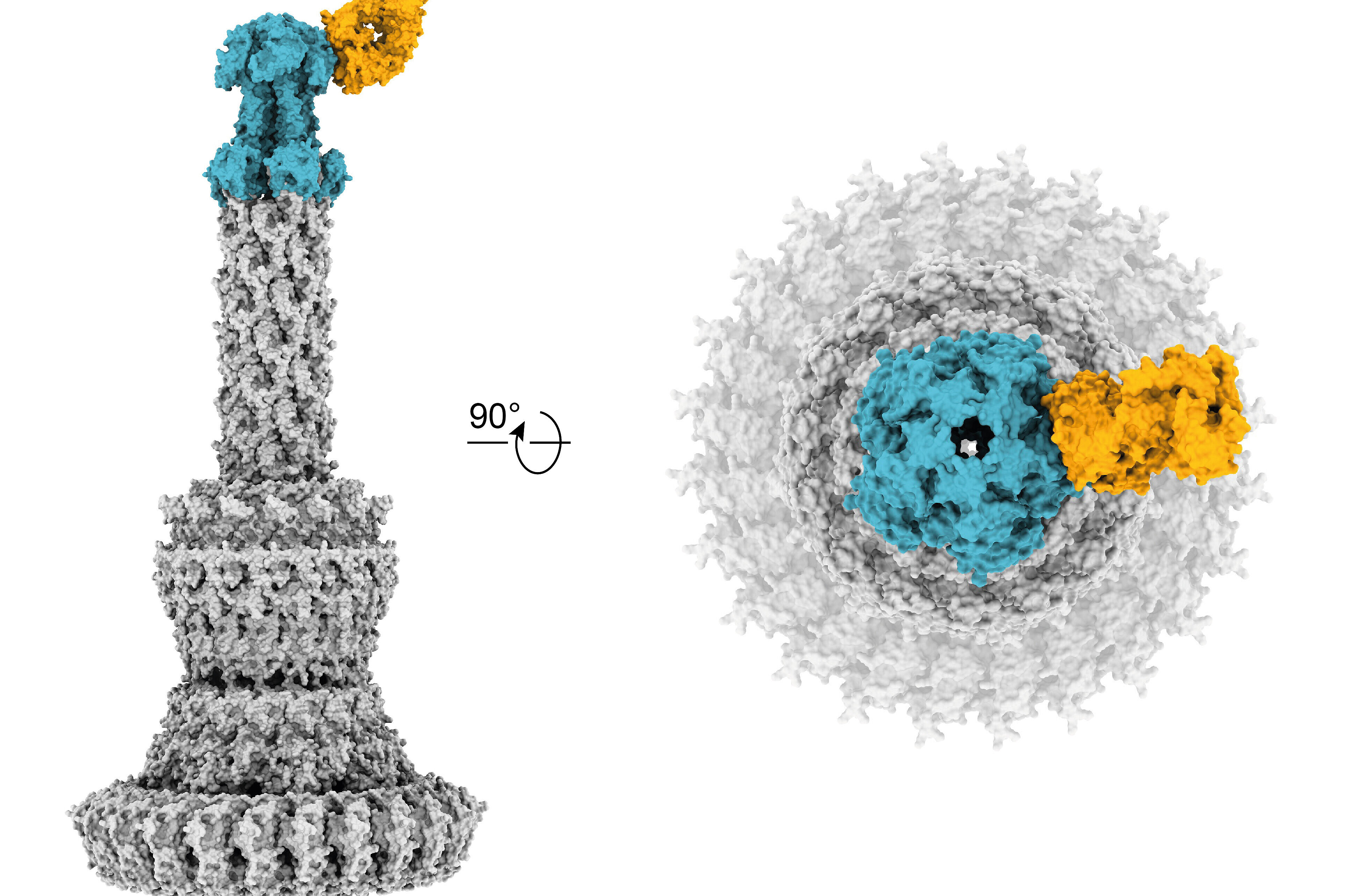
Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., ... , Rox K., Hilgenfeld R.
(2020)
Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors
Science,
368
(6489)
Author count: 22
Blockus S., Sake S.M., Wetzke M., Grethe C., Graalmann T., Pils M., Le Goffic R., Galloux M., Prochnow H., Rox K., Huttel S., Rupcic Z., Wiegmann B., Dijkman R., Rameix-Welti M.A., Eleouet J.F., Duprex W.P., Thiel V., Hansen G., Brönstrup M., ... , Haid S., Pietschmann T.
(2020)
Labyrinthopeptins as virolytic inhibitors of respiratory syncytial virus cell entry
Antiviral Res.,
177
(May)
Author count: 21
Le P., Kunold E., Macsics R., Rox K., Jennings M.C., Ugur I., Reinecke M., Chaves-Moreno D., Hackl M.W., Fetzer C., Mandl F.A.M., Lehmann J., Korotkov V.S., Hacker S.M., Kuster B., Antes I., Pieper D.H., Rohde M., Wuest W.M., ... , Medina E., Sieber S.A.
(2020)
Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms
Nat.Chem.,
12
(2)
Author count: 24
Prochnow H., Rox K., Birudukota N.V.S., Weichert L., Hotop S.K., Klahn P., Mohr K., Franz S., Banda D.H., Blockus S., Schreiber J., Haid S., Oeyen M., Martinez J.P., Süssmuth R.D., Wink J., Meyerhans A., Goffinet C., Messerle M., Schulz T.F., Kröger A., Schols D., ... , Pietschmann T., Brönstrup M.
(2020)
Labyrinthopeptins exert broad-spectrum antiviral activity through lipid-binding-mediated virolysis
J.Virol.,
94
(2)
Author count: 13
Sommer R., Rox K., Wagner S., Hauck D., Henrikus S.S., Newsad S., Arnold T., Ryckmans T., Brönstrup M., Imberty A., Varrot A., ... , Hartmann R.W., Titz A.
(2019)
Anti-biofilm Agents against Pseudomonas aeruginosa: A Structure-Activity Relationship Study of C-Glycosidic LecB Inhibitors
J.Med.Chem.,
62
(20)
Author count: 15
Klahn P., Fetz V., Ritter A., Collisi W., Hinkelmann B., Arnold T., Tegge W., Rox K., Hüttel S., Mohr K.I., Wink J., Stadler M., Wissing J., ... , Jänsch L., Brönstrup M.
(2019)
The nuclear export inhibitor aminoratjadone is a potent effector in extracellular-targeted drug conjugates
Chem.Sci.,
10
(20)
Author count: 8
Heck J.G., Rox K., Lünsdorf H., Lückerath T., Klaassen N., Medina E., ... , Goldmann O., Feldmann C.
(2018)
Zirconyl Clindamycinphosphate Antibiotic Nanocarriers for Targeting Intracellular Persisting Staphylococcus aureus
ACS Omega,
3
(8)
Author count: 14
Sommer R., Wagner S., Rox K., Varrot A., Hauck D., Wamhoff E.C., Schreiber J., Ryckmans T., Brunner T., Rademacher C., Hartmann R.W., Brönstrup M., ... , Imberty A., Titz A.
(2018)
Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas aeruginosa
J.Am.Chem.Soc.,
140
(7)
Author count: 8
Rox K., Jansen R., Loof T.G., Gillen C.M., Bernecker S., Walker M.J., ... , Chhatwal G.S., Müller R.
(2017)
Linoleic and palmitoleic acid block streptokinase-mediated plasminogen activation and reduce severity of invasive group A streptococcal infection
Sci.Rep.,
7
(1)
Author count: 4
Rox K., Rohde M., Chhatwal G.S., Müller R.
(2017)
Disorazoles Block Group A Streptococcal Invasion into Epithelial Cells Via Interference with the Host Factor Ezrin
Cell Chem.Biol.,
24
(2)
















![[Translate to English:] Gut bacterium Escherichia coli](/fileadmin/_processed_/1/9/csm_hzi_wiss_ecoli_mr_006__1__a674b3dd36.jpg)