Pathways in Infection and Nociception

Our Research
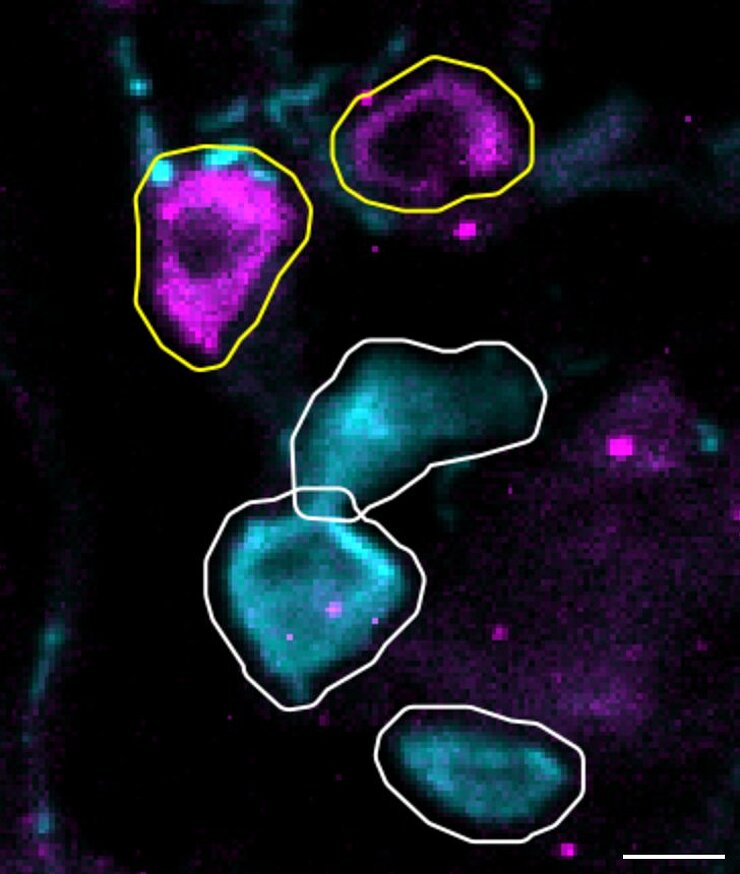
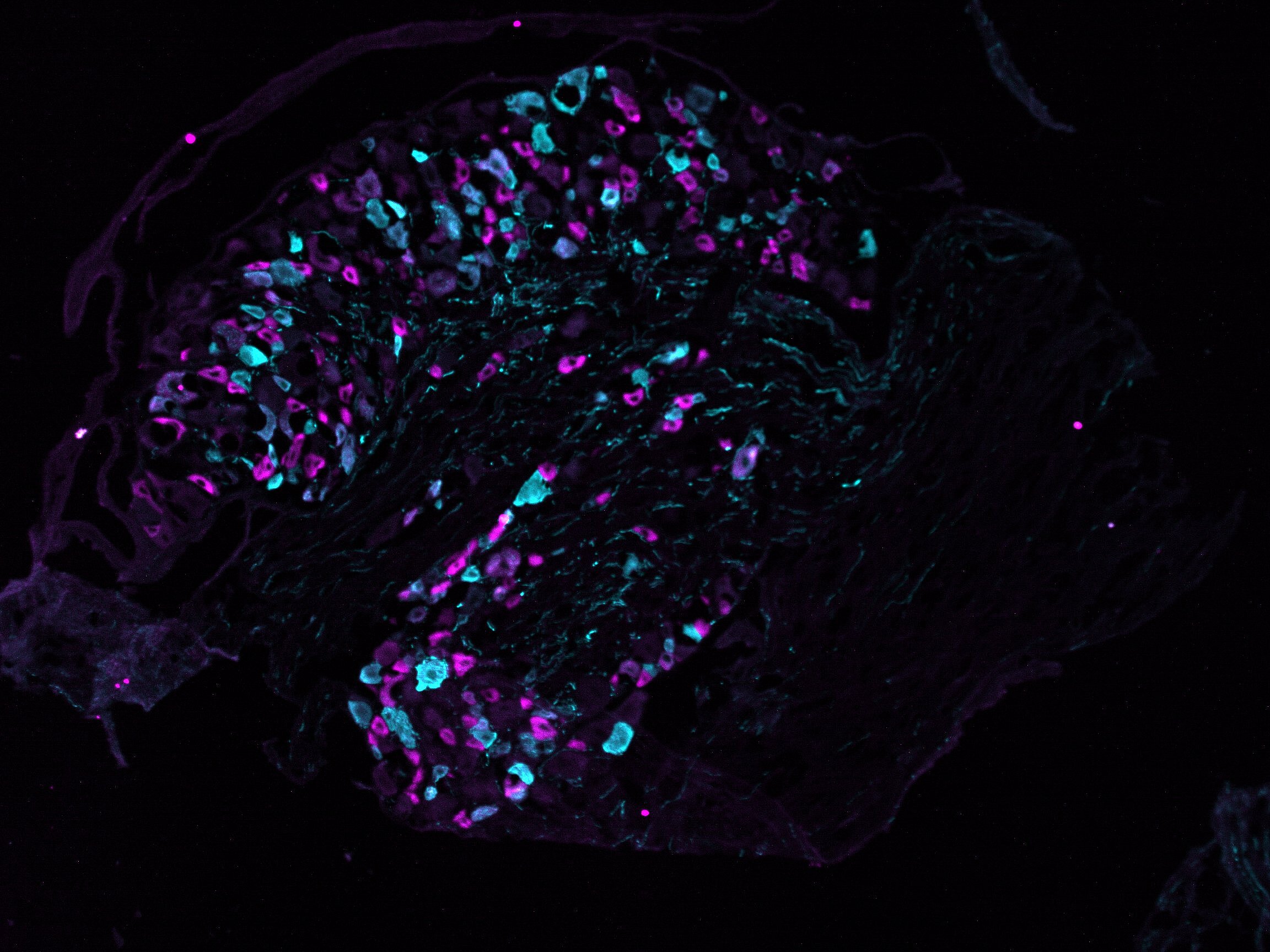
Infectious diseases pose a major threat to human health, as highlighted by the COVID-19 pandemic. Pain, a protective mechanism signaling harm, is often associated with infections either directly or through immune-mediated pathways. Pain commonly arises from hyperactivity in peripheral nerves, particularly small-to-medium sensory neurons (nociceptors) located in the dorsal root ganglia (DRG). Inflammatory mediators released during infection activate nociceptors and trigger pain. For example, myalgia was a prominent symptom of COVID-19 and increased the risk of chronic pain. Herpes simplex viruses (HSV), on the other hand, are neurotropic and induce pain directly through mechanisms that remain unclear. HSV-1 infects an estimated 3.8 billion people globally and causes painful oral blisters. Varicella zoster virus, following chickenpox, remains latent in DRGs and reactivates as shingles in about 30 per cent of people. Shingles leads to painful skin rashes, with many patients developing post-herpetic neuralgia—a condition poorly managed by current therapies. Understanding pain pathways in infection biology is critical but remains severely understudied.
Our research leverages cutting-edge electrophysiology, ultra-sensitive proteomics, behavioral phenotyping, and cell culture methodology to investigate the following:
- We have recently mapped the subset-specific proteome of mouse sensory neurons. Since proteins relate directly to function, we now ask: how does this diversity shape responses to pathogens using a variety of mouse models? Which subsets of sensory neurons are susceptible to viral infections, and why? Is the host response viral strain–specific?
- We will develop novel behavioral pipelines capable of assessing non-evoked mouse pain and itch behavior in response to pathogens, as well as following analgesic applications.
- We will define the functional and proteomic diversity of human sensory neuron subtypes to identify molecules with a higher chance of clinical success.
Our vision is to pioneer the field of “nocifection”; to generate a comprehensive mechanistic understanding of pain during infections, with an emphasis on viral infections.
Our Research
Infectious diseases pose a major threat to human health, as highlighted by the COVID-19 pandemic. Pain, a protective mechanism signaling harm, is often associated with infections either directly or through immune-mediated pathways. Pain commonly arises from hyperactivity in peripheral nerves, particularly small-to-medium sensory neurons (nociceptors) located in the dorsal root ganglia (DRG). Inflammatory mediators released during infection activate nociceptors and trigger pain. For example, myalgia was a prominent symptom of COVID-19 and increased the risk of chronic pain. Herpes simplex viruses (HSV), on the other hand, are neurotropic and induce pain directly through mechanisms that remain unclear. HSV-1 infects an estimated 3.8 billion people globally and causes painful oral blisters. Varicella zoster virus, following chickenpox, remains latent in DRGs and reactivates as shingles in about 30 per cent of people. Shingles leads to painful skin rashes, with many patients developing post-herpetic neuralgia—a condition poorly managed by current therapies. Understanding pain pathways in infection biology is critical but remains severely understudied.
Our research leverages cutting-edge electrophysiology, ultra-sensitive proteomics, behavioral phenotyping, and cell culture methodology to investigate the following:
- We have recently mapped the subset-specific proteome of mouse sensory neurons. Since proteins relate directly to function, we now ask: how does this diversity shape responses to pathogens using a variety of mouse models? Which subsets of sensory neurons are susceptible to viral infections, and why? Is the host response viral strain–specific?
- We will develop novel behavioral pipelines capable of assessing non-evoked mouse pain and itch behavior in response to pathogens, as well as following analgesic applications.
- We will define the functional and proteomic diversity of human sensory neuron subtypes to identify molecules with a higher chance of clinical success.
Our vision is to pioneer the field of “nocifection”; to generate a comprehensive mechanistic understanding of pain during infections, with an emphasis on viral infections.
Sampurna Chakrabarti
„We seek to identify mechanisms involved in infection-related pain, one cell at a time”

Originally from Kolkata, India, Sampurna Chakrabarti earned a BS in Biological Sciences (Neuroscience track) and a BA in Psychology from the University at Buffalo, NY, USA. She then received a Gates Cambridge Scholarship to pursue her PhD at the University of Cambridge, UK, where she investigated mechanisms of peripheral sensitization in inflammatory knee pain and won multiple research awards, including a research stay at the European Molecular Biology Laboratory in Rome, Italy.
In 2020, Sam joined the Max Delbrück Center for Molecular Medicine in Berlin, Germany, as a postdoctoral scientist studying mechanosensation and diversity of sensory neurons, supported by an Alexander von Humboldt Fellowship. During this time, she also secured several independent research grants, including the Grass Fellowship at the Marine Biological Laboratory, MA, USA, where she examined pain-like behavior in sharks.
Sam has published over 10 papers in leading journals, served as a reviewer for several reputed journals including PAIN and Science Advances, and is an Early Career Editorial Fellow at the Journal of Pain. Since October 2025, she has been leading the HUMAN-funded junior research group “Pathways in Infection and Nociception” (PAIN) at the HZI to study infection-induced changes in nerve function leading to pain in both mice and humans.
Selected Publications
- Chakrabarti, S. et al. Ultrasensitive proteomics uncovers nociceptor diversity and novel pain targets. bioRxiv 2025.09.20.677124 (2025) DOI: 10.1101/2025.09.20.677124 (Preprint).
- Chakrabarti, S. et al. Touch sensation requires the mechanically gated ion channel ELKIN1. Science 383, 992–998 (2024). DOI: 10.1126/science.adl0495
- Chakrabarti, S. et al. Intraarticular adeno‐associated virus serotype AAV‐PHP. S–mediated chemogenetic targeting of knee‐innervating dorsal root ganglion neurons alleviates inflammatory pain in mice. Arthritis Rheumatol. 72, 1749–1758 (2020). DOI: 10.1002/art.41314
- Chakrabarti, S., Jadon, D. R., Bulmer, D. C. & Smith, E. S. J. Human osteoarthritic synovial fluid increases excitability of mouse dorsal root ganglion sensory neurons: an in-vitro translational model to study arthritic pain. Rheumatology 59, 662–667 (2020). DOI: 10.1093/rheumatology/kez331
- Chakrabarti, S. et al. Sensitization of knee-innervating sensory neurons by tumor necrosis factor-α-activated fibroblast-like synoviocytes: an in vitro, coculture model of inflammatory pain. Pain 161, 2129 (2020). DOI: 10.1097/j.pain.0000000000001890
Publications
A complete list of publications can be found here.
Newsroom
Are you interested in a bachelor or master thesis? We are looking forward to your request!