Biomolecular Condensates in Infection

Our Research
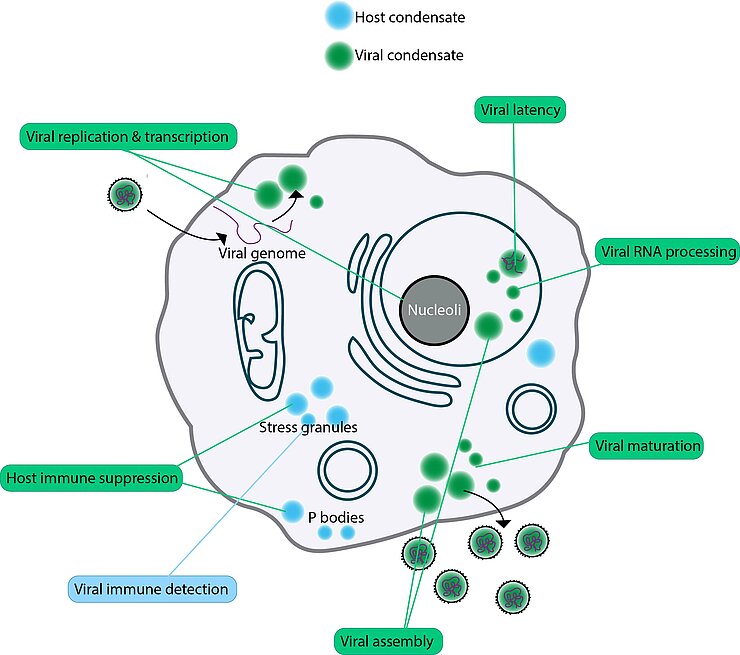
Our research focuses on the role of biomolecular condensates in pathogen-host interactions, with a long-term goal of developing innovative antivirals. Condensates, comprising biological polymers like nucleic acids and proteins, form concentrated droplets within cells through a process known as phase separation. Recent studies emphasize their importance in viral replication, capsid assembly, packaging, and immune evasion. While host cells use condensates as part of their defense, viruses often exploit or deactivate these structures to facilitate infection. Given the relevance and ubiquitous use of biomolecular condensates in viral replication, they represent promising targets for antivirals.
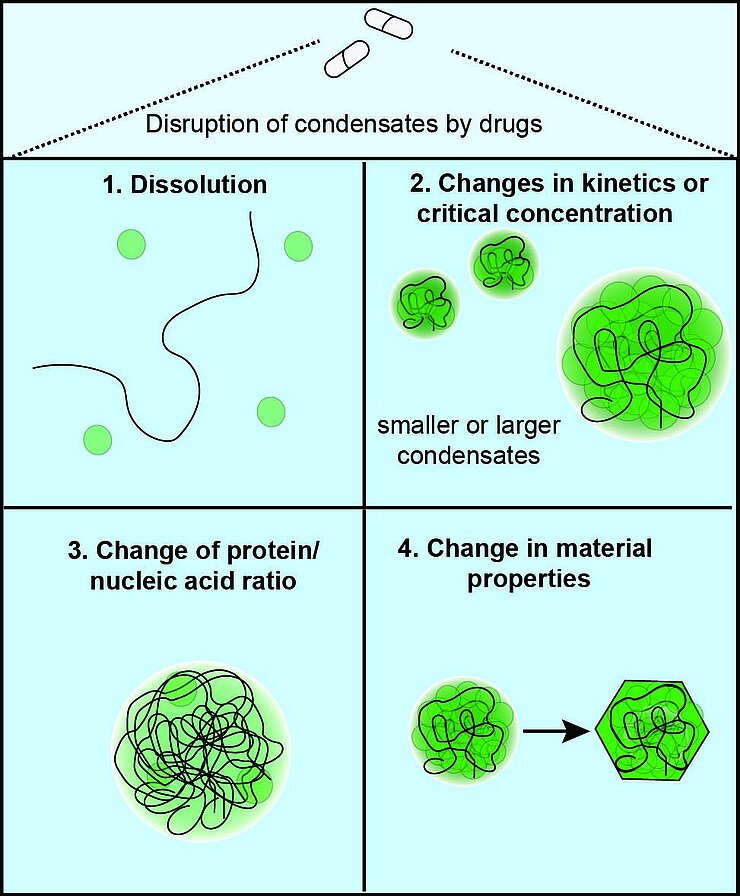
Furthermore, viruses exhibit remarkable mutation rates and adaptability, leading to resistance against existing antivirals. Consequently, novel therapeutic strategies are urgently needed. Addressing multi-component condensates, rather than individual drug targets, could thus be an effective way to reduce viral resistance to treatments, particularly when host condensates are involved.
The central theme of our lab is to combine fundamental research on biomolecular condensates in virology with drug discovery. We combine advanced imaging, biochemical, and innovative organoid techniques to study fundamental principles of host-viral interactions. Once drug targets have been identified, we employ high-throughput and automation techniques for compound screening in close collaboration with chemists at HZI.
Two pillars of research:
- Fundamental Research: Investigate host-viral condensate interactions using simple in vitro and more complex organoid models.
- Drug Discovery: Discover viral and host condensates as potential drug targets.
Our Research
Our research focuses on the role of biomolecular condensates in pathogen-host interactions, with a long-term goal of developing innovative antivirals. Condensates, comprising biological polymers like nucleic acids and proteins, form concentrated droplets within cells through a process known as phase separation. Recent studies emphasize their importance in viral replication, capsid assembly, packaging, and immune evasion. While host cells use condensates as part of their defense, viruses often exploit or deactivate these structures to facilitate infection. Given the relevance and ubiquitous use of biomolecular condensates in viral replication, they represent promising targets for antivirals.
Furthermore, viruses exhibit remarkable mutation rates and adaptability, leading to resistance against existing antivirals. Consequently, novel therapeutic strategies are urgently needed. Addressing multi-component condensates, rather than individual drug targets, could thus be an effective way to reduce viral resistance to treatments, particularly when host condensates are involved.
The central theme of our lab is to combine fundamental research on biomolecular condensates in virology with drug discovery. We combine advanced imaging, biochemical, and innovative organoid techniques to study fundamental principles of host-viral interactions. Once drug targets have been identified, we employ high-throughput and automation techniques for compound screening in close collaboration with chemists at HZI.
Two pillars of research:
- Fundamental Research: Investigate host-viral condensate interactions using simple in vitro and more complex organoid models.
- Drug Discovery: Discover viral and host condensates as potential drug targets.
Dr. Christiane Iserman
„We are just beginning to uncover the ubiquitous roles of biomolecular condensates in viral infections”

Driven by her interest in science, Christiane Iserman studied Electro- and Information Technology and Biology and was selected to join the Biology PLUS/International Program at the University of Düsseldorf, which included a year of research at Michigan State University. She was awarded the “Deutschlandstipendium” for three consecutive years. Following her B.Sc., she entered the IMPRS PhD program at the Max Planck Institute of Molecular Cell Biology with a fellowship for a fast-track PhD. During her PhD under Prof. Simon Alberti, she investigated how cells measure stress signal intensity and adjust their response. She demonstrated how yeast proteins condense in response to stress, triggering downstream gene expression through translational regulation. This work, now published in Cell, was a key achievement during her academic training.
She later pursued postdoctoral research at UNC Chapel Hill with Prof. Amy Gladfelter, where she explored RNA-based condensation in viral packaging and was recognized with a Christiane Nüsslein-Volhard fellowship. Her work that was published in Molecular Cell and recognized with a NIH Fast Grants Award for its impact on coronavirus biology.
As Head of Condensate Biology for Infectious Diseases at Dewpoint Therapeutics, she led cross-disciplinary teams working in oncology, nephrology, and virology, advancing programs from hypothesis generation to high-throughput screening (HTS) and hit-to-lead processes. Her collaborative work with Pharma partners honed her expertise in drug discovery. At the Max Planck Institute of Molecular Cell Biology and Genetics, she later on worked with Anne Grapin-Botton and Tony Hyman to set-up organoid systems for condensate biology research.
Since October 1, 2025, Christiane Iserman has headed the HUMAN (Human Microbe Alliance for Universal Health) junior research group “Biomolecular Condensates in Infection” at the Helmholtz Centre for Infection Research (HZI) in Braunschweig. Her group is dedicated to the research of biomolecular condensates in infection in order to develop innovative antiviral strategies.
Team



Selected Publications
Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production., Iserman, C., Desroches Altamirano, C., Jegers, C., Friedrich, U., Zarin, T., Fritsch, A. W., Mittasch, M., Domingues, A., Hersemann, L., Jahnel, M., Richter, D., Guenther, U. P., Hentze, M. W., Moses, A. M., Hyman, A. A., Kramer, G., Kreysing, M., Franzmann, T. M. and Alberti, S., Cell, 181, 818-831 e819 DOI: 10.1016/j.cell.2020.04.009
Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid., Iserman, C., Roden, C., Boerneke, M., Sealfon, R., McLaughlin, G., Jungreis, I., Park, C., Boppana, A., Fritch, E., Hou, Y. J., Theesfeld, C., Troyanskaya, O. G., Baric, R. S., Sheahan, T. P., Weeks, K. and Gladfelter, A. S., Molecular Cell, DOI: 10.1016/j.molcel.2020.11.041.
Non-invasive perturbations of intracellular flow reveal physical principles of cell organization., Mittasch, M., Gross, P., Nestler, M., Fritsch, A. W., Iserman, C., Kar, M., Munder, M., Voigt, A., Alberti, S., Grill, S. W. and Kreysing, M., Nat Cell Biol, 20(3) 344-351. DOI: 10.1038/s41556-017-0032-9
Double-stranded RNA drives SARS-CoV-2 nucleocapsid protein to undergo phase separation at specific temperatures., Roden, C., Dai, Y., Giannetti, K., Seim, I., Lee, M., Sealfon, R., McLaughlin, G., Boerneke, M., Iserman, C., Wey, S., Ekena, J., Troyanskaya, O., Weeks, K., YOu, L., Chilkoti, A., Gladfelter, A.S., Nucleic Acid Research, DOI: 10.1093/nar/gkac596.
Biomolecular Condensates as Novel Antiviral Targets., Martin, E.W., Iserman, C., Olety, B., Mitrea, D.M., Klein, I.A., JMB, DOI: 10.1016/j.jmb.2023.168380.
Publications
A complete list of publications can be found here.
Newsroom
Are you interested in a bachelor or master thesis? We are looking forward to your request!