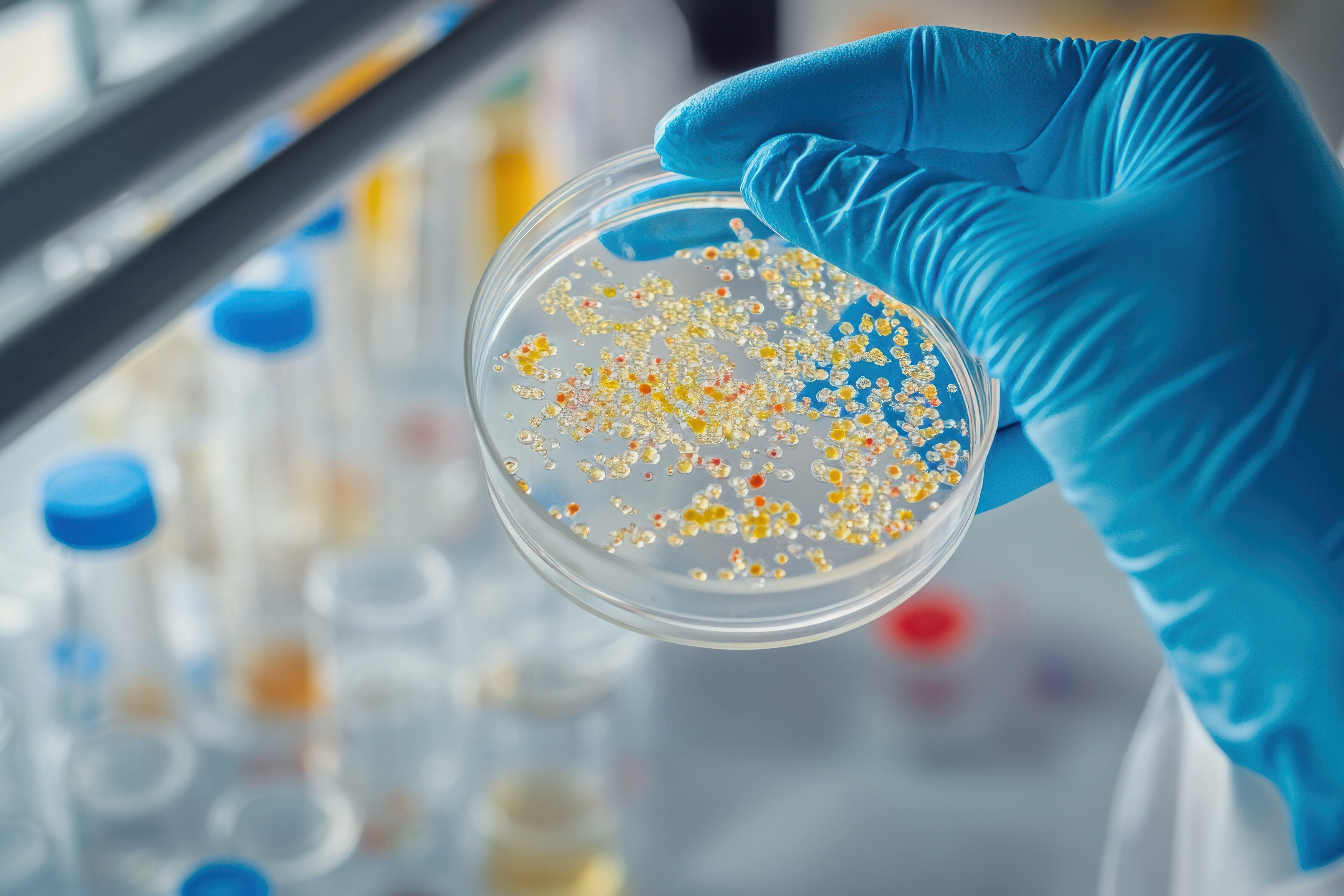
CorA is a natural product anti-infective with a unique mechanism of action, showing potent activity against filarial infections such as river blindness (onchocerciasis) and lymphatic filariasis (LF), as well as against Staphylococci and the bacteria that cause community acquired pneumonia (CAP). The filarial infections are predominantly found in Africa (and in the case of LF, also in Asia), affect millions of patients and are caused by parasitic worms that depend on bacterial symbionts. CorA selectively targets these symbionts, offering a promising therapeutic approach. The active substance was identified by scientists at the HZI in the bacterium Corallococcus coralloides in the 1980s.
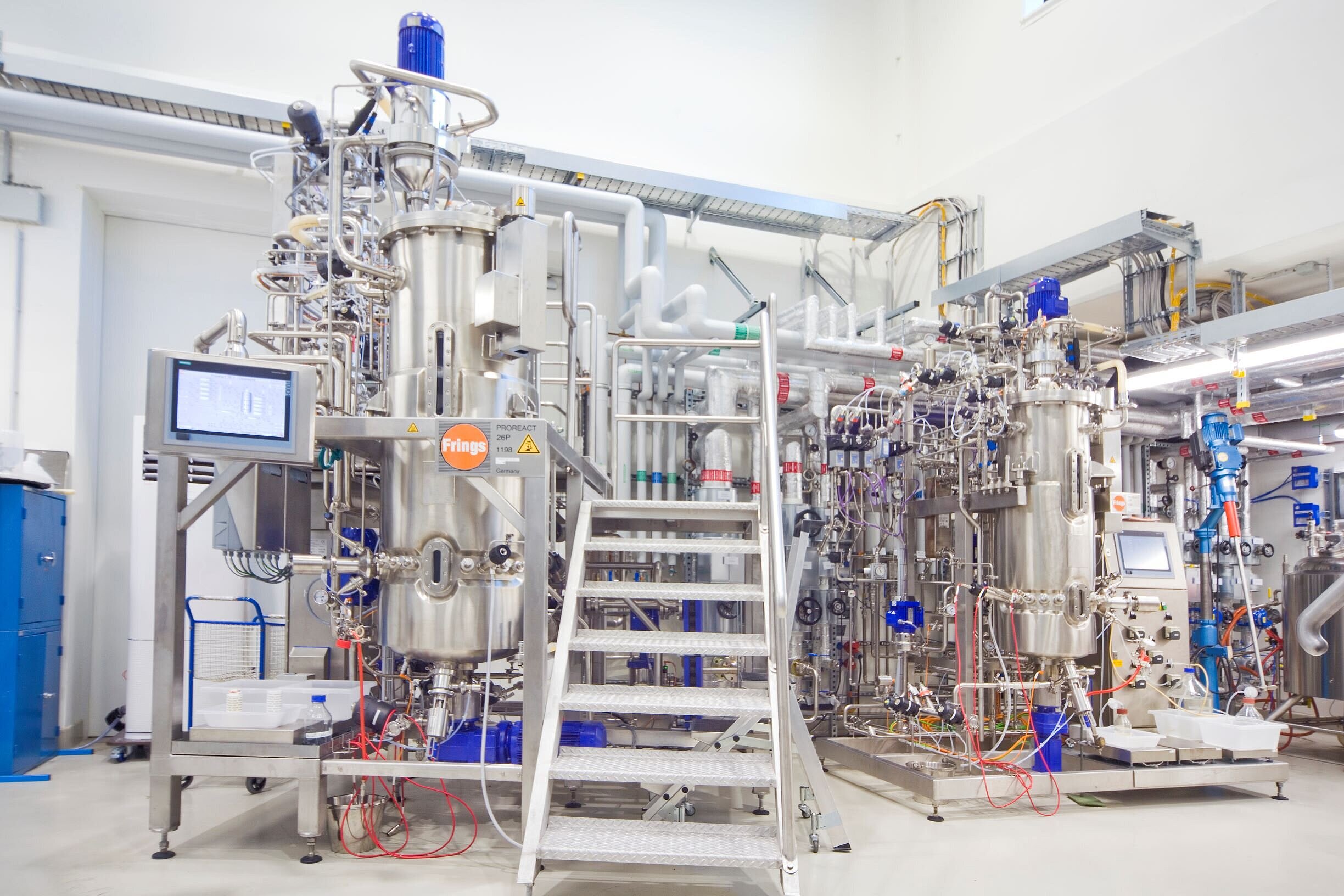
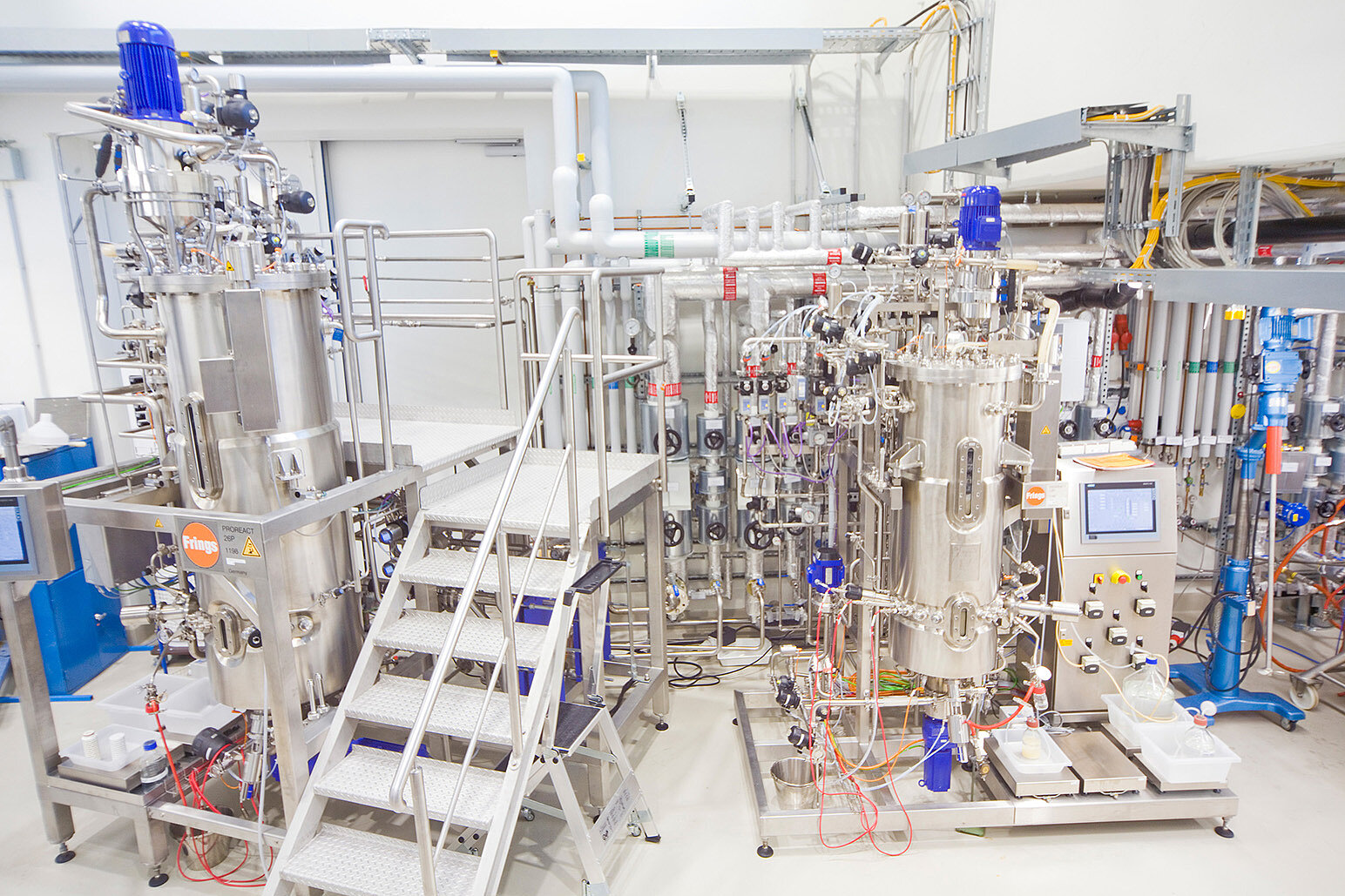
The technology transfer was successfully completed at the 1500 L scale at Phyton Biotech’s GMP facilities near Hamburg, Germany (Fermentation) and in Vancouver, Canada (Purification). Key outcomes include:
- Robust fermentation of the genetically engineered Myxococcus xanthus strain.
- Efficient cell separation using tangential flow filtration (TFF).
- High-yield resin capture and purification of CorA, meeting all critical quality attributes.
“Completing the Corallopyronin A technology transfer marks an important milestone for Phyton Biotech and our partners,” said Colin Marr, President of Phyton Biotech. “It highlights our comprehensive fermentation expertise including organism classes not broadly used in industry and our ability to support the scale-up of this novel therapy for clinical evaluation.”
With the initial transfer process now complete, Phyton Biotech is preparing to demonstrate scalability and process performance at the 7500 L scale to supply GMP quality CorA for upcoming clinical trials.
This project is part of a broader international collaboration supported by the DZIF and the Global Health Innovative Technology (GHIT) Fund from Japan through collaboration with Eisai Co. Ltd., aiming to advance CorA as a new treatment option for diseases that disproportionately affect underserved populations. Prof. Achim Hoerauf, principal investigator of the CorA development consortium and Director of the Institute for Medical Microbiology, Immunology and Parasitology emphasizes the importance of the DZIF and GHIT in enabling the project to meet high international standards in preclinical development. “Through the DZIF and GHIT, we were able to implement these standards within a consortium of academic institutions working alongside industrial partners—an achievement that would normally take years,” says Hoerauf..
The DZIF’s broad spectrum of expertise was key to the project’s success: the optimized heterologous production strain was developed at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), a site of the HZI in cooperation with Saarland University, under the leadership of Prof. Rolf Müller. The production process was established by Prof. Marc Stadler, head of the department “Microbial Drugs” at HZI, while orally available formulations were developed in Bonn under Prof. Karl Wagner (supported by the ForTra gGmbH for Research Transfer of the Else Kröner Fresenius Foundation) and will now be used in the upcoming clinical trial.
![Dr Charlotte Schwenner [Translate to English:] Charlotte Schwenner](/fileadmin/_processed_/6/9/csm_Charlotte_Schwenner_7952cfe0a7.webp)