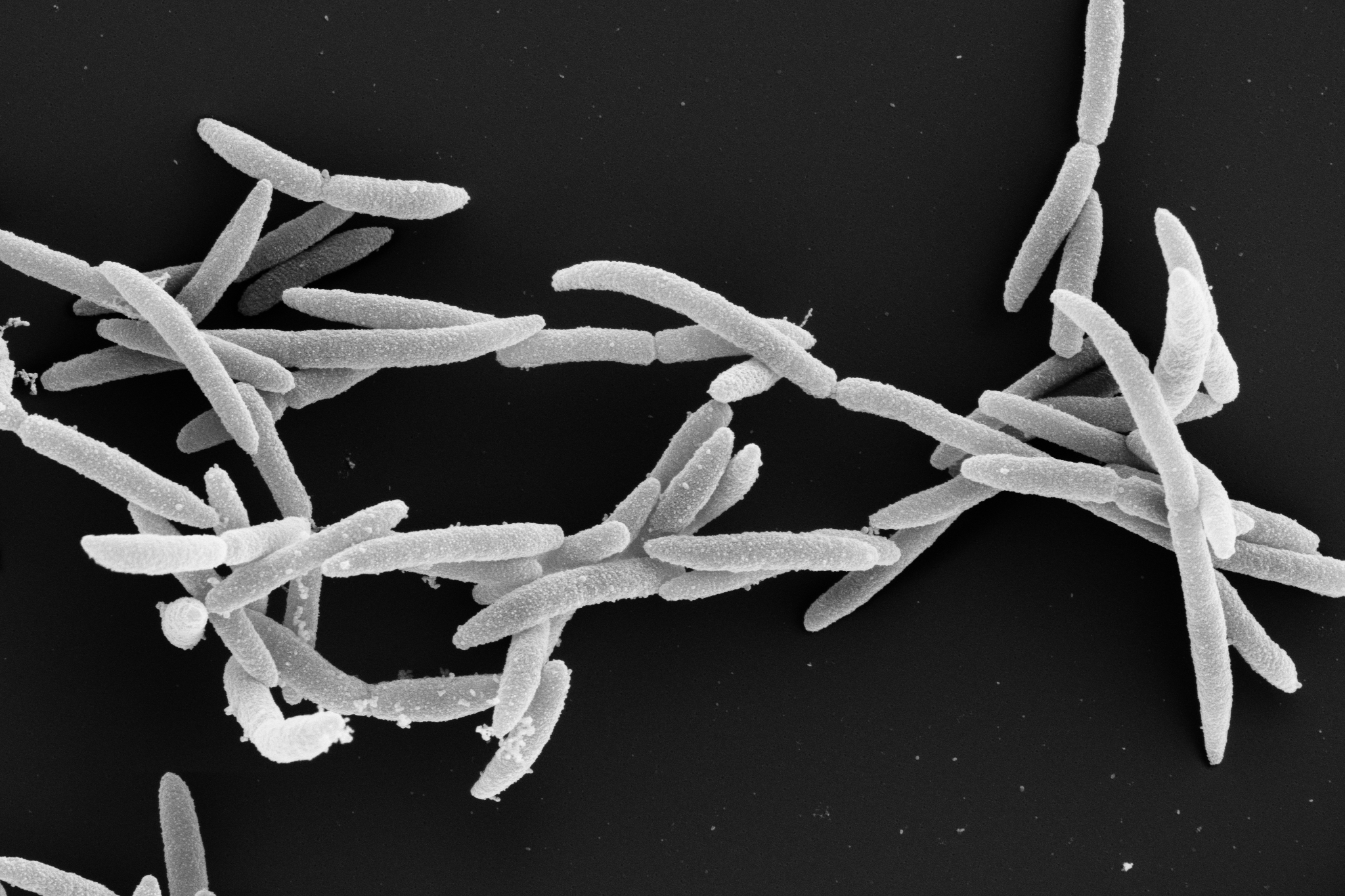
Understanding how human-specific bacteria make us sick is challenging. In a new study published today in Nature Genetics, researchers show that it is now possible to use cultured mini-organs, so-called organoids, to map how bacteria colonize the human intestinal mucosa. The team at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität Würzburg (JMU), and at Uppsala University, focused specifically on Shigella flexneri. The bacterium causes severe intestinal inflammation in humans and is responsible for many deaths per year, particularly among young children.
“Our results provide a more realistic picture of the factors that influence Shigella colonization in our intestines. This could enable new treatment approaches in the future,” says Lars Barquist, an associated scientist at HIRI and professor at the University of Toronto in Canada, and co-senior author of the study. “For the first time, we have been able to map the genes Shigella needs to cause infection using a human model that mimics intestinal tissue. The study also demonstrates that cultured human mini-organs can now be used to investigate a variety of serious infections, particularly those for which the lack of laboratory animal models has previously limited research,” adds Maria Letizia Di Martino from Uppsala University, who led development of the experimental system.
Intestinal models derived from stem cells
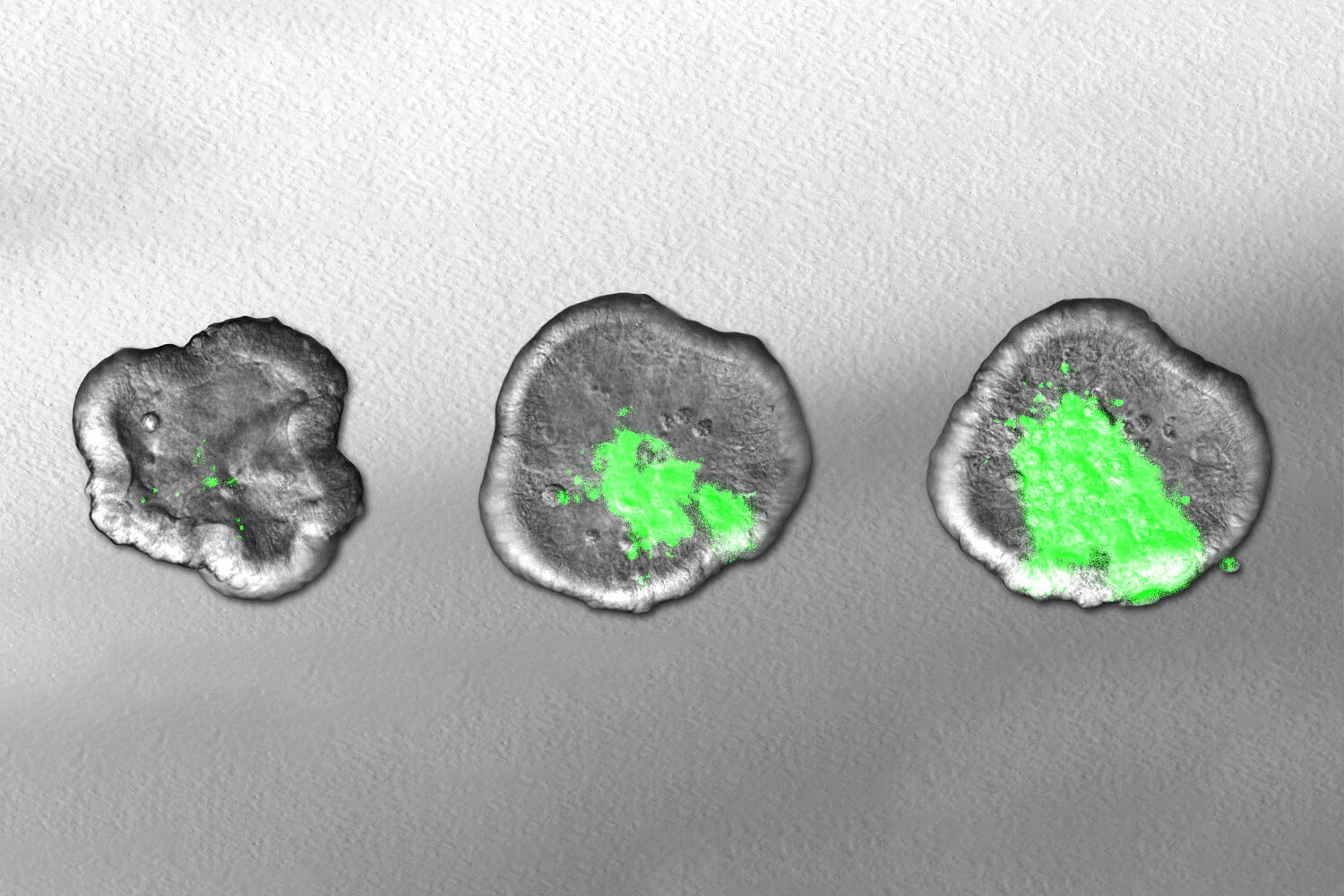
Shigella bacteria are invasive pathogens that attack the body’s tissues using a variety of ‘weapons’ to invade the intestinal mucosa and manipulate the body’s immune system functions. In the current study, the researchers focused on identifying the genes responsible for producing these weapons. To do this, they generated intestinal organoids—miniature intestinal models—grown from human stem cells purified from surgical waste material. They then used a method that randomly knocks out bacterial genes and tested how these changes affected Shigella’s ability to infect the human intestinal model.
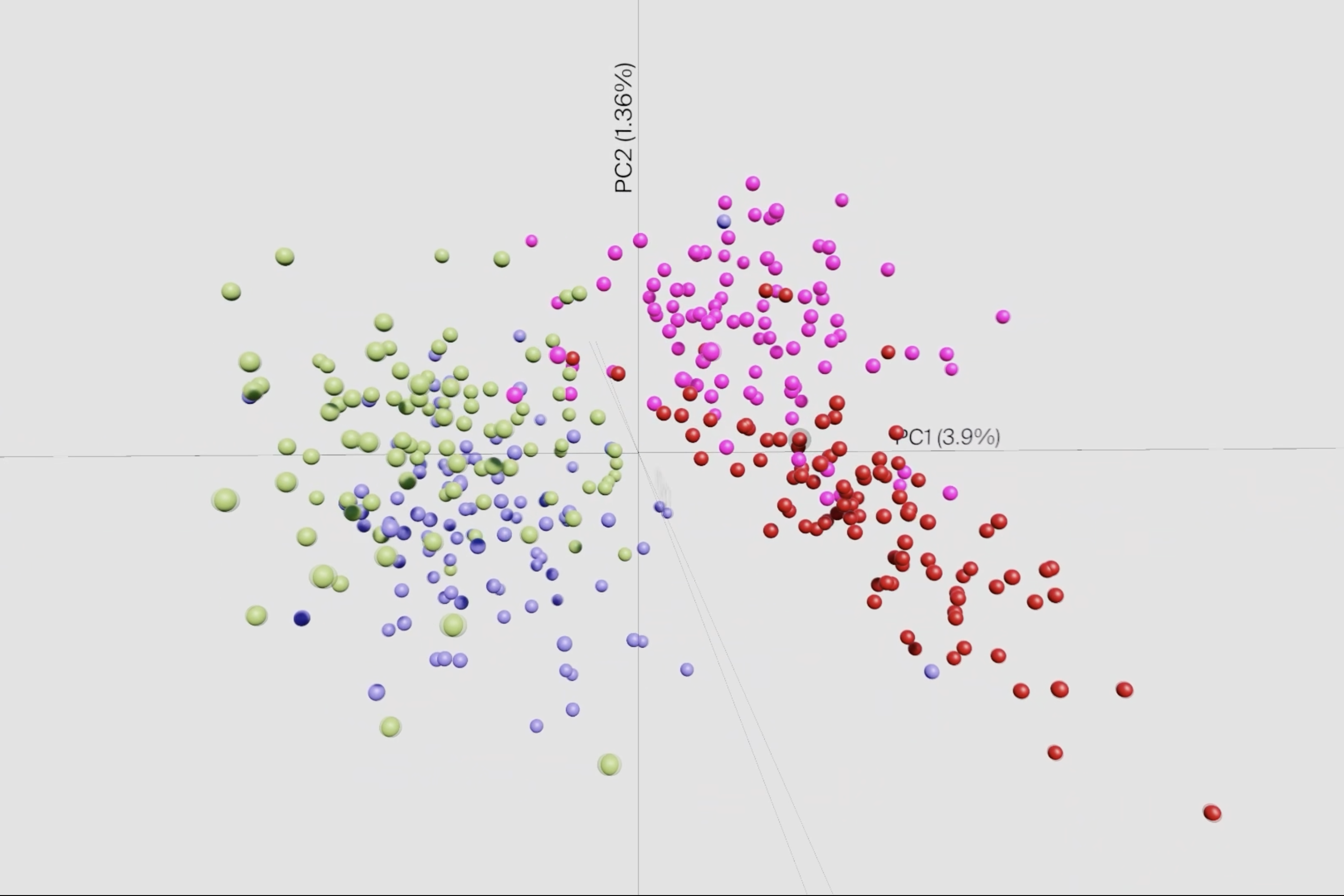
One of the biggest challenges in conducting such screening is the occurrence of population bottlenecks. These arise when factors such as nutrient deficiency or competition for colonization sites temporarily decrease the number of bacteria, regardless of the fitness of individual mutants. This leads to a stochastic loss of mutants, which can distort the results of large screens. To compensate for this, the team has developed a statistical model that integrates information across a large number of small-scale experiments to generate a genome-wide map. This enabled the team to generate the first comprehensive map of the genes Shigella uses to invade human intestinal tissue.
Particularly surprising was the discovery that certain changes in transfer ribonucleic acids (tRNAs) can control the activity of the type III secretion system – a complex apparatus that bacteria use to unleash their disease-causing properties. Such systems cost bacteria a lot of energy to produce, which is why they control them very precisely. “It was previously known that bacteria do this, for example, by switching off foreign DNA or by regulating the number of certain plasmids, small circular DNA molecules. This study now reveals a third, fundamental mechanism by which this control can be achieved,” explains Laura Jenniches, the postdoctoral researcher in the Barquist lab who led statistical and computational analysis.
“Shigella has around 5,000 genes, but we found that only about 100 of them are necessary for the bacterium to colonize tissue and cause aggressive infection. This list is a goldmine for understanding the progression of infections and for developing new treatments that can ‘turn off’ the bacteria’s pathogenic behavior,” says Mikael Sellin from Uppsala University, another of the study’s senior authors. The screening technology used is transferable to many other pathogens and will enable realistic studies on human tissue in the future. Lars Barquist looks ahead: “This study lays the technical groundwork needed to investigate a wide variety of pathogens in these realistic organoid models that capture key aspects of human physiology in the lab.”