In the course of evolution, bacteria have learned to adapt to adverse living conditions like hardly any other type of organism. It is therefore not surprising that the natural products they produce cover a broad spectrum of biological activities. One group of natural products with a particularly broad spectrum of activity are the relatively understudied PoTeMs (polycyclic tetramate macrolactams). The first known representative of this group is ikarugamycin, which was discovered in 1972 and is effective against both bacteria and protozoa. The development of PoTeMs into potential drugs has so far been hindered, as they could only be produced in a very laborious and costly process and offered only few opportunities for structural modification. In three research papers, a research team led by Tobias Gulder, head of the HIPS department Natural Product Biotechnology, has now successfully addressed these issues. In a first study, the team established an efficient biotechnological process for producing large quantities of PoTeMs such as ikarugamycin. In a follow-up study, they developed a genetic “plug-and-play system” that allows oxygen to be introduced at selected positions of the PoTeM structures, thereby specifically modifying their properties. To further exploit the pharmaceutical potential of PoTeMs, the researchers pursued chemo-enzymatic and molecular biological approaches to successfully modify their core structure for the first time. The team has now published the results of this third study in the journal Angewandte Chemie International Edition. The HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University.
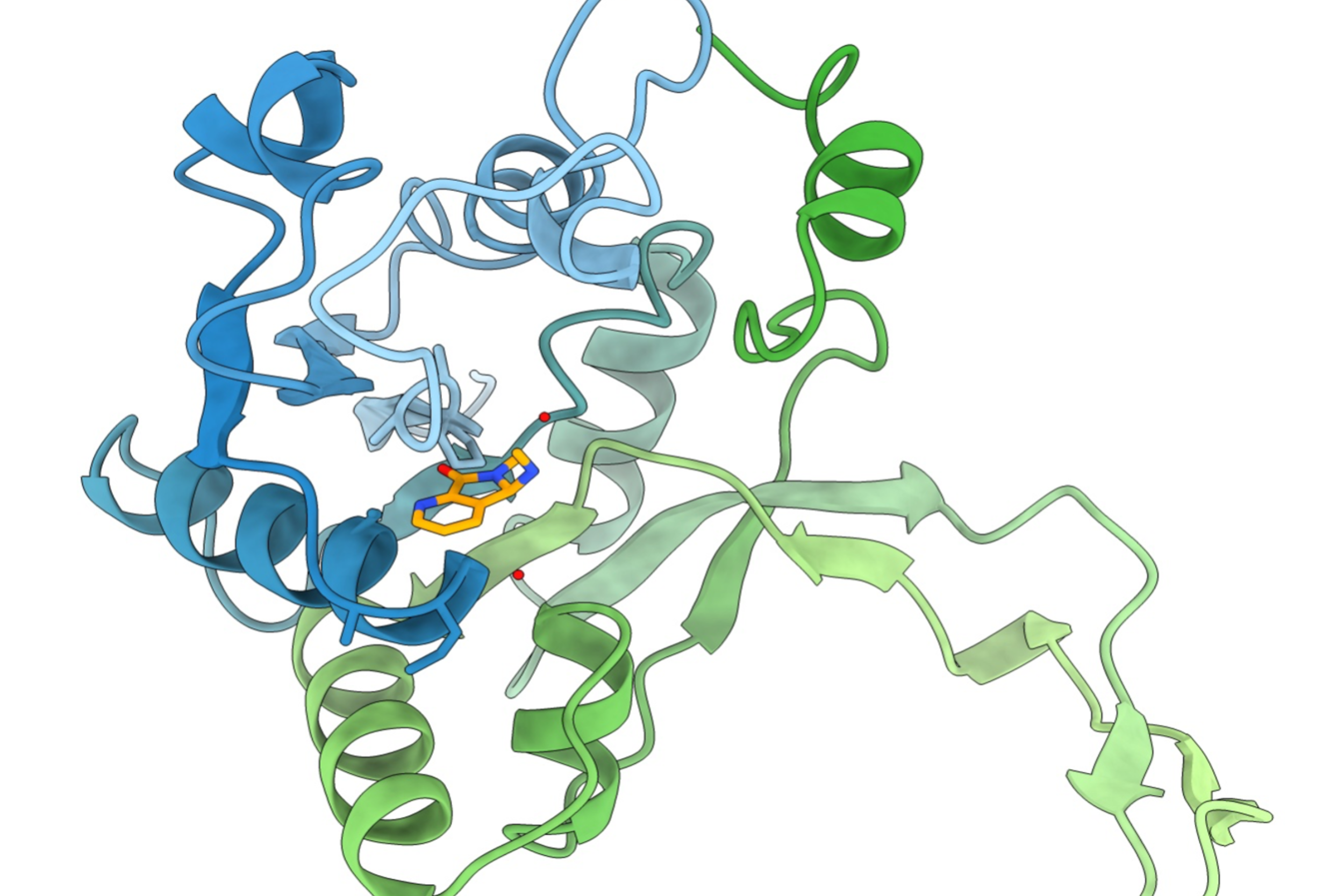
“The bacterial synthesis of ikarugamycin can be roughly divided into two steps,” says Sebastian Schuler, who was involved in all three studies. ”First, the precursor molecule lysobacterene A is produced by the enzyme complex IkaA, which uses acetate and the amino acid ornithine as substrates. Lysobacterene A is then converted by the PoTeM cyclases IkaB and IkaC (IkaBC in short) into a ring-shaped molecule – ikarugamycin. When we isolated IkaBC in the laboratory and examined them more closely, we found that the cyclases can convert not only lysobacterene A but also similar molecules that we had previously chemically synthesized.” Subsequently, the team used this strategy to produce a previously unknown PoTeM natural product, called homo-ikarugamycin. To do this, the researchers used a synthetic precursor whose molecular scaffold is extended by one carbon as compared to Lysobacterene A.
Due to the laborious production of the precursor, the team went one step further: “Instead of chemically accomplishing the first half of the synthesis of homo-ikarugamycin, we asked ourselves whether we could modify IkaA so that it also uses longer substrates to produce lysobacterene A,” says Tobias Gulder. “To this end, we genetically modified the part of IkaA that is responsible for selecting the amino acid building block so that it accepts the amino acid lysine, which is one carbon unit longer than ornithine. This allowed us to trick IkaA into producing the previously synthesized precursor itself, which was then converted into homo-ikarugamycin by IkaBC. The best part is that all these steps take place in the living bacterium.” The production of homo-ikarugamycin by bacteria is not only significantly easier than its chemical synthesis, but also offers the advantage of being more cost-effective and requiring fewer resources.
In the future, the established technologies will be used as a basis to produce further new PoTeMs. To this end, the modified version of IkaA could, for example, be combined with cyclases other than IkaBC. In the long term, Gulder and his team want to exploit the pharmaceutical potential of PoTeMs and develop promising molecules into active ingredients for the treatment of infectious diseases. Tobias Gulder has been a department head at HIPS and a professor at Saarland University since the end of 2023, but so far he has been conducting research at TU Dresden. His team will relocate to Saarbrücken in the course of the year.


![[Translate to English:] 3D-kontrastierte Oberflächenstruktur von LasB (beige) mit einem Inhibitor der 3. Generation (dunkelblau), der Zink (grau) bindet.](/fileadmin/_processed_/8/b/csm_LasB_Foto_b7734ae7a4.png)