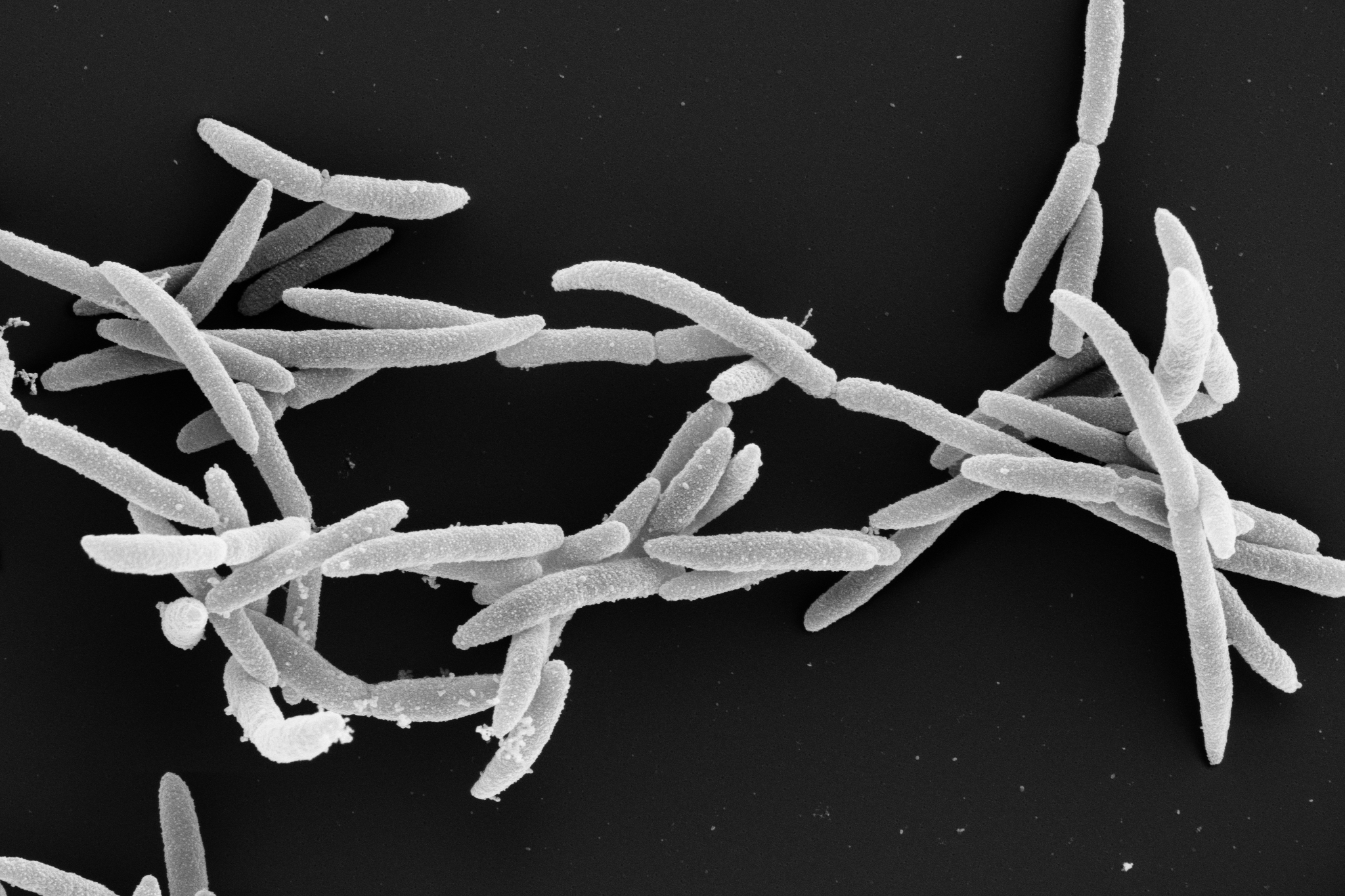
The human oral microbiome consists of over 700 bacterial species from seven distinct phyla, including Fusobacterium nucleatum. However, this microbe is not limited to the oral cavity. It can also colonize other parts of our body—particularly tumor tissues in esophageal, colon, and breast cancer. There, Fusobacterium nucleatum has been linked to tumor growth and metastasis. Targeted inhibition of these and other fusobacteria could improve recovery prospects for cancer patients. But how can this be achieved? Scientists at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität Würzburg (JMU), have set out to answer this question.
“Fusobacteria have long been overlooked despite their clinical importance,” says Jörg Vogel, Director of the HIRI and corresponding author of the study. “One of the goals of my research group at the HIRI is to explore strategies that can specifically eliminate these bacteria in carcinomas.”
Custom-made antibiotics
Although conventional antibiotics can inhibit fusobacteria and thereby slow down tumor growth, their long-term use can cause undesirable side effects such as gastrointestinal problems due to a disrupted gut flora. This is because they attack not only harmful but also beneficial bacteria. To avoid these risks, scientists at the HIRI are developing new, targeted treatment strategies. In their current study, published the American Society for Microbiology journal mBio, they focus on peptide nucleic acid, or PNA. These are synthetic molecules similar to DNA or RNA. Unlike natural nucleic acids, the backbone of PNAs consists of a peptide-like structure instead of sugar and phosphate groups, which gives PNAs exceptional stability. The bases remain similar to those in DNA, allowing PNAs to target transcripts. As antisense molecules, PNAs bind to complementary messenger RNA (mRNA), blocking its function and inhibiting the production of essential proteins. This targeted action positions PNAs as a potential new generation of antibacterial agents.
An unexpected discovery
Surprisingly, the antisense molecules introduced to target specific genes failed to inhibit bacterial growth. However, the research team made an unexpected discovery: the control compound, FUS79, which did not target a specific transcript, exhibited strong activity against five fusobacterial strains without affecting other tested bacterial species. “The result was surprising because the compound does not work in the expected way for antisense nucleic acid chains, but instead has a novel mechanism,” explains Valentina Cosi, first author of the study and a doctoral student in Jörg Vogel's laboratory. “It seems to exert its effects by inducing membrane stress, destabilizing the bacterial cell membrane or impairing its function, though we still need to investigate this in more detail.” Vogel adds that “our next step is to decipher the very mechanism of this compound and optimize it further to enhance its effectiveness”.
The study lays the groundwork for the development of antisense therapeutics against Fusobacterium nucleatum and highlights the potential of this compound as a new strategy for more targeted antibiotics. The gained insights have the potential to accelerate further research in this field, which could ultimately improve cancer treatment outcomes.