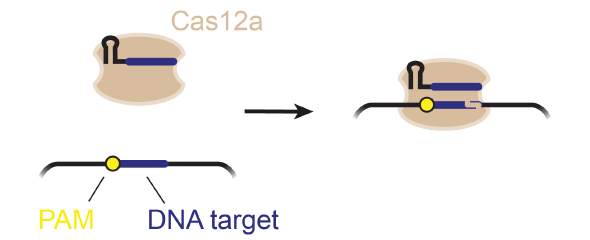
“The presence of a suitable PAM remains a barrier for directed gene editing,” says Prof Chase Beisel, head of the HIRI research group “RNA Synthetic Biology”. This has led researchers to mine the existing diversity of Cas nucleases in bacteria and archaea for ones that recognize new and unique PAMs. In his current study, Beisel collaborated with researchers from North Carolina State University and the biotech company Benson Hill to characterize the PAMs recognized by a set of technologically important Cas nucleases called Cas12a. They found that two of the characterized nucleases recognized quite distinct PAMs despite the nucleases being almost identical. Furthermore, when the researchers started mutating one nuclease to look like the other, they uncovered a variant that recognized different PAMs. This nuclease also exhibited more efficient genome editing than either of the original nucleases.
These technologies are also becoming cheap and rapid diagnostics, including for the detection of SARS-CoV-2.
When looking for new and interesting Cas nucleases, researchers would normally evaluate nucleases that are extremely different from each other, with the assumption that similar nucleases behave similarly. “Our work challenges this notion with the surprising finding that similar nucleases can in fact behave quite differently,” says Beisel. Building on this finding, the researchers argue for a deeper mining of nucleases in nature and for mutating existing nucleases to change the PAM. The nuclease mutant with enhanced activity and a unique PAM that was discovered in this study would also be beneficial for CRISPR technologies by allowing Cas12a nucleases to access even more potential DNA targets.
CRISPR technologies are actively applied for gene therapy to combat genetic diseases and for immunotherapy to combat cancer. “These technologies are also becoming cheap and rapid diagnostics, including for the detection of SARS-CoV-2. Our work helps expand the number of potential target sites and thus the flexibility in how these technologies can be applied,” says Beisel. His research group will continue to explore the natural diversity of CRISPR nucleases and CRISPR-Cas immune systems in hopes of revealing new and interesting functions that could bring about the next revolution in CRISPR technologies.
Original publication:
Thomas Jacobsen, Fani Ttofali, Chunyu Liao, Srinivas Manchalu, Benjamin N Gray, Chase L Beisel: Characterization of Cas12a nucleases reveals diverse PAM profiles between closely-related orthologs. Nucleic Acids Research. 2020; DOI: 10.1093/nar/gkaa272